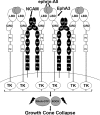
The Neural Cell Adhesion Molecule (NCAM) Promotes Clustering and Activation of EphA3 Receptors in GABAergic Interneurons to Induce Ras Homolog Gene Family, Member A (RhoA)/Rho-associated protein kinase (ROCK)-mediated Growth Cone Collapse
- PMID: 27803162
- PMCID: PMC5159490
- DOI: 10.1074/jbc.M116.760017
The Neural Cell Adhesion Molecule (NCAM) Promotes Clustering and Activation of EphA3 Receptors in GABAergic Interneurons to Induce Ras Homolog Gene Family, Member A (RhoA)/Rho-associated protein kinase (ROCK)-mediated Growth Cone Collapse
Abstract
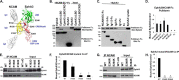
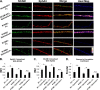
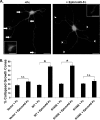
Establishment of a proper balance of excitatory and inhibitory connectivity is achieved during development of cortical networks and adjusted through synaptic plasticity. The neural cell adhesion molecule (NCAM) and the receptor tyrosine kinase EphA3 regulate the perisomatic synapse density of inhibitory GABAergic interneurons in the mouse frontal cortex through ephrin-A5-induced growth cone collapse. In this study, it was demonstrated that binding of NCAM and EphA3 occurred between the NCAM Ig2 domain and EphA3 cysteine-rich domain (CRD). The binding interface was further refined through molecular modeling and mutagenesis and shown to be comprised of complementary charged residues in the NCAM Ig2 domain (Arg-156 and Lys-162) and the EphA3 CRD (Glu-248 and Glu-264). Ephrin-A5 induced co-clustering of surface-bound NCAM and EphA3 in GABAergic cortical interneurons in culture. Receptor clustering was impaired by a charge reversal mutation that disrupted NCAM/EphA3 association, emphasizing the importance of the NCAM/EphA3 binding interface for cluster formation. NCAM enhanced ephrin-A5-induced EphA3 autophosphorylation and activation of RhoA GTPase, indicating a role for NCAM in activating EphA3 signaling through clustering. NCAM-mediated clustering of EphA3 was essential for ephrin-A5-induced growth cone collapse in cortical GABAergic interneurons, and RhoA and a principal effector, Rho-associated protein kinase, mediated the collapse response. This study delineates a mechanism in which NCAM promotes ephrin-A5-dependent clustering of EphA3 through interaction of the NCAM Ig2 domain and the EphA3 CRD, stimulating EphA3 autophosphorylation and RhoA signaling necessary for growth cone repulsion in GABAergic interneurons in vitro, which may extend to remodeling of axonal terminals of interneurons in vivo.
Keywords: EphA3; NCAM; Ras homolog gene family, member A (RhoA); growth cone collapse; interneuron; neurobiology; neurodevelopment; oligomerization; synaptic plasticity.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Maness P. F., and Schachner M. (2007) Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat. Neurosci. 10, 19–26 - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

