The Role of Hydrogen Sulfide in Renal System
- PMID: 27803669
- PMCID: PMC5067532
- DOI: 10.3389/fphar.2016.00385
The Role of Hydrogen Sulfide in Renal System
Abstract
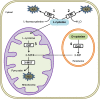
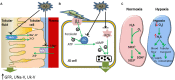
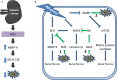
Hydrogen sulfide has gained recognition as the third gaseous signaling molecule after nitric oxide and carbon monoxide. This review surveys the emerging role of H2S in mammalian renal system, with emphasis on both renal physiology and diseases. H2S is produced redundantly by four pathways in kidney, indicating the abundance of this gaseous molecule in the organ. In physiological conditions, H2S was found to regulate the excretory function of the kidney possibly by the inhibitory effect on sodium transporters on renal tubular cells. Likewise, it also influences the release of renin from juxtaglomerular cells and thereby modulates blood pressure. A possible role of H2S as an oxygen sensor has also been discussed, especially at renal medulla. Alternation of H2S level has been implicated in various pathological conditions such as renal ischemia/reperfusion, obstructive nephropathy, diabetic nephropathy, and hypertensive nephropathy. Moreover, H2S donors exhibit broad beneficial effects in renal diseases although a few conflicts need to be resolved. Further research reveals that multiple mechanisms are underlying the protective effects of H2S, including anti-inflammation, anti-oxidation, and anti-apoptosis. In the review, several research directions are also proposed including the role of mitochondrial H2S in renal diseases, H2S delivery to kidney by targeting D-amino acid oxidase/3-mercaptopyruvate sulfurtransferase (DAO/3-MST) pathway, effect of drug-like H2S donors in kidney diseases and understanding the molecular mechanism of H2S. The completion of the studies in these directions will not only improves our understanding of renal H2S functions but may also be critical to translate H2S to be a new therapy for renal diseases.
Keywords: H2S; acute kidney injury; chronic kidney disease; diabetic nephropathy; hydrogen sulfide; renal physiology.
Figures
Similar articles
-
The roles of hydrogen sulfide in renal physiology and disease states.Ren Fail. 2022 Dec;44(1):1289-1308. doi: 10.1080/0886022X.2022.2107936. Ren Fail. 2022. PMID: 35930288 Free PMC article. Review.
-
Hydrogen Sulfide: Recent Progression and Perspectives for the Treatment of Diabetic Nephropathy.Molecules. 2019 Aug 6;24(15):2857. doi: 10.3390/molecules24152857. Molecules. 2019. PMID: 31390847 Free PMC article. Review.
-
Hydrogen Sulfide and the Kidney: Physiological Roles, Contribution to Pathophysiology, and Therapeutic Potential.Antioxid Redox Signal. 2022 Feb;36(4-6):220-243. doi: 10.1089/ars.2021.0014. Epub 2021 Dec 31. Antioxid Redox Signal. 2022. PMID: 34978847 Review.
-
Chemistry of Hydrogen Sulfide-Pathological and Physiological Functions in Mammalian Cells.Cells. 2023 Nov 22;12(23):2684. doi: 10.3390/cells12232684. Cells. 2023. PMID: 38067112 Free PMC article. Review.
-
Hydrogen sulfide in renal physiology, disease and transplantation--the smell of renal protection.Nitric Oxide. 2015 Apr 30;46:37-49. doi: 10.1016/j.niox.2015.01.005. Epub 2015 Feb 2. Nitric Oxide. 2015. PMID: 25656225 Review.
Cited by
-
A Bioluminescent Probe for H2S Detection in Tumor Microenvironment.ACS Bio Med Chem Au. 2025 Jan 3;5(1):175-183. doi: 10.1021/acsbiomedchemau.4c00102. eCollection 2025 Feb 19. ACS Bio Med Chem Au. 2025. PMID: 39990954 Free PMC article.
-
International Union of Basic and Clinical Pharmacology. CII: Pharmacological Modulation of H2S Levels: H2S Donors and H2S Biosynthesis Inhibitors.Pharmacol Rev. 2017 Oct;69(4):497-564. doi: 10.1124/pr.117.014050. Pharmacol Rev. 2017. PMID: 28978633 Free PMC article. Review.
-
Hydrogen Sulfide Attenuates Renin Angiotensin and Aldosterone Pathological Signaling to Preserve Kidney Function and Improve Exercise Tolerance in Heart Failure.JACC Basic Transl Sci. 2018 Dec 31;3(6):796-809. doi: 10.1016/j.jacbts.2018.08.011. eCollection 2018 Dec. JACC Basic Transl Sci. 2018. PMID: 30623139 Free PMC article.
-
Hydrogen Sulfide Biology and Its Role in Cancer.Molecules. 2022 May 25;27(11):3389. doi: 10.3390/molecules27113389. Molecules. 2022. PMID: 35684331 Free PMC article. Review.
-
Effect of Hydrogen Sulfide on Essential Functions of Polymorphonuclear Leukocytes.Toxins (Basel). 2023 Mar 4;15(3):198. doi: 10.3390/toxins15030198. Toxins (Basel). 2023. PMID: 36977089 Free PMC article.
References
-
- Ahmad A., Olah G., Szczesny B., Wood M. E., Whiteman M., Szabo C. (2016). AP39, A mitochondrially targeted hydrogen sulfide donor, exerts protective effects in renal epithelial cells subjected to oxidative stress in vitro and in acute renal injury in vivo. Shock 45 88–97. 10.1097/SHK.0000000000000478 - DOI - PMC - PubMed
-
- Ahmad F. U., Sattar M. A., Rathore H. A., Abdullah M. H., Tan S., Abdullah N. A., et al. (2012). Exogenous hydrogen sulfide (H2S) reduces blood pressure and prevents the progression of diabetic nephropathy in spontaneously hypertensive rats. Ren. Fail. 34 203–210. 10.3109/0886022X.2011.643365 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

