Roles of FGF Signals in Heart Development, Health, and Disease
- PMID: 27803896
- PMCID: PMC5067508
- DOI: 10.3389/fcell.2016.00110
Roles of FGF Signals in Heart Development, Health, and Disease
Abstract
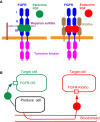
The heart provides the body with oxygen and nutrients and assists in the removal of metabolic waste through the blood vessels of the circulatory system. It is the first organ to form during embryonic morphogenesis. FGFs with diverse functions in development, health, and disease are signaling proteins, mostly as paracrine growth factors or endocrine hormones. The human/mouse FGF family comprises 22 members. Findings obtained from mouse models and human diseases with FGF signaling disorders have indicated that several FGFs are involved in heart development, health, and disease. Paracrine FGFs including FGF8, FGF9, FGF10, and FGF16 act as paracrine signals in embryonic heart development. In addition, paracrine FGFs including FGF2, FGF9, FGF10, and FGF16 play roles as paracrine signals in postnatal heart pathophysiology. Although FGF15/19, FGF21, and FGF23 are typical endocrine FGFs, they mainly function as paracrine signals in heart development or pathophysiology. In heart diseases, serum FGF15/19 levels or FGF21 and FGF23 levels decrease or increase, respectively, indicating their possible roles in heart pathophysiology. FGF2 and FGF10 also stimulate the cardiac differentiation of cultured stem cells and cardiac reprogramming of cultured fibroblasts. These findings provide new insights into the roles of FGF signaling in the heart and potential therapeutic strategies for cardiac disorders.
Keywords: FGF; biomarker; development; differentiation; disease; heart.
Figures

References
-
- Abu-Issa R., Smyth G., Smoak I., Yamamura K., Meyers E. N. (2002). Fgf8 is required for pharyngeal arch and cardiovascular development in the mouse. Development 129, 4613–4625. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

