Efficacy of phosphodiesterase-4 inhibitors in juvenile Batten disease (CLN3)
- PMID: 27804148
- PMCID: PMC5215570
- DOI: 10.1002/ana.24815
Efficacy of phosphodiesterase-4 inhibitors in juvenile Batten disease (CLN3)
Abstract
Objective: Juvenile neuronal ceroid lipofuscinosis (JNCL), or juvenile Batten disease, is a pediatric lysosomal storage disease caused by autosomal recessive mutations in CLN3, typified by blindness, seizures, progressive cognitive and motor decline, and premature death. Currently, there is no treatment for JNCL that slows disease progression, which highlights the need to explore novel strategies to extend the survival and quality of life of afflicted children. Cyclic adenosine monophosphate (cAMP) is a second messenger with pleiotropic effects, including regulating neuroinflammation and neuronal survival. Here we investigated whether 3 phosphodiesterase-4 (PDE4) inhibitors (rolipram, roflumilast, and PF-06266047) could mitigate behavioral deficits and cell-specific pathology in the Cln3Δex7/8 mouse model of JNCL.
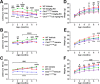
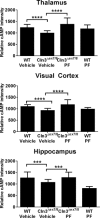
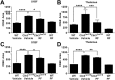
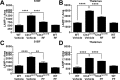
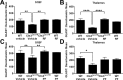
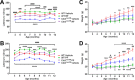
Methods: In a randomized, blinded study, wild-type (WT) and Cln3Δex7/8 mice received PDE4 inhibitors daily beginning at 1 or 3 months of age and continuing for 6 to 9 months, with motor deficits assessed by accelerating rotarod testing. The effect of PDE4 inhibitors on cAMP levels, astrocyte and microglial activation (glial fibrillary acidic protein and CD68, respectively), lysosomal pathology (lysosomal-associated membrane protein 1), and astrocyte glutamate transporter expression (glutamate/aspartate transporter) were also examined in WT and Cln3Δex7/8 animals.
Results: cAMP levels were significantly reduced in the Cln3Δex7/8 brain, and were restored by PF-06266047. PDE4 inhibitors significantly improved motor function in Cln3Δex7/8 mice, attenuated glial activation and lysosomal pathology, and restored glutamate transporter expression to levels observed in WT animals, with no evidence of toxicity as revealed by blood chemistry analysis.
Interpretation: These studies reveal neuroprotective effects for PDE4 inhibitors in Cln3Δex7/8 mice and support their therapeutic potential in JNCL patients. Ann Neurol 2016;80:909-923.
© 2016 The Authors Annals of Neurology published by Wiley Periodicals, Inc. on behalf of American Neurological Association.
Figures


References
-
- International Batten Disease Consortium . Isolation of a novel gene underlying Batten disease, CLN3. Cell 1995;82:949–957. - PubMed
-
- Williams RE, Mole SE. New nomenclature and classification scheme for the neuronal ceroid lipofuscinoses. Neurology 2012;79:183–191. - PubMed
-
- Autti T, Hamalainen J, Aberg L, et al. Thalami and corona radiata in juvenile NCL (CLN3): a voxel‐based morphometric study. Eur J Neurol 2007;14:447–450. - PubMed
-
- Autti TH, Hamalainen J, Mannerkoski M, et al. JNCL patients show marked brain volume alterations on longitudinal MRI in adolescence. J Neurol 2008;255:1226–1230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

