Neoadjuvant chemotherapy for Patients with advanced epithelial ovarian cancer: A Meta-Analysis
- PMID: 27804983
- PMCID: PMC5090201
- DOI: 10.1038/srep35914
Neoadjuvant chemotherapy for Patients with advanced epithelial ovarian cancer: A Meta-Analysis
Abstract
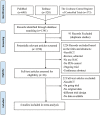
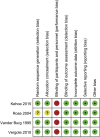
The value of neoadjuvant chemotherapy (NAC) has not yet been fully defined. We aimed to systematically evaluate the influence of neoadjuvant chemotherapy (NAC) on survival and complete cytoreduction after debulking surgery in advanced epithelial ovarian cancer (AEOC) patients. We searched PubMed, Embase, and the Cochrane Central Register of Controlled Trials for the randomized controlled trials (RCTs) comparing NAC and primary debulking surgery (PDS) in AEOC patients. The last search date is February 25, 2016. Cochrane systematic evaluation was used to evaluate bias risk of included studies. RevMan 5.3 software was used for statistical analysis. A total of 4 RCTs involving 1922 patients were included. Compared with PDS, NAC may contribute to the completeness of debulking removal [no residual disease (RR: 2.37; 95%CI: 1.94-2.91; P<0.00001), residual disease ≤1 cm (RR: 1.28; 95%CI: 1.04-1.57; P = 0.02), optimal cytoreduction rate (RR: 1.76; 95%CI: 1.57-1.98; P<0.00001)], but there were no significant differences in both groups with regard to overall survival (HR: 0.94; 95%Cl: 0.81-1.08; P = 0.38) and progression-free survival (HR: 0.89; 95%Cl: 0.77-1.03; P = 0.12). This meta-analysis indicates that the higher rate of optimal debulking made NAC more favorable as a treatment option for AEOC patients with non-inferior survival compared with PDS.
Figures
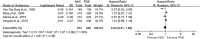
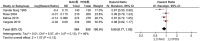

Similar articles
-
Does neoadjuvant chemotherapy plus cytoreductive surgery improve survival rates in patients with advanced epithelial ovarian cancer compared with cytoreductive surgery alone?J BUON. 2015 Mar-Apr;20(2):580-7. J BUON. 2015. PMID: 26011353
-
Does neoadjuvant chemotherapy plus cytoreductive surgery improve survival rates in patients with advanced epithelial ovarian cancer compared with cytoreductive surgery alone?J BUON. 2015 May-Jun;20(3):847-54. J BUON. 2015. PMID: 26214639
-
Only complete tumour resection after neoadjuvant chemotherapy offers benefit over suboptimal debulking in advanced ovarian cancer.Eur J Obstet Gynecol Reprod Biol. 2017 Dec;219:100-105. doi: 10.1016/j.ejogrb.2017.10.019. Epub 2017 Oct 19. Eur J Obstet Gynecol Reprod Biol. 2017. PMID: 29078115
-
Appropriate Recommendations for Surgical Debulking in Stage IV Ovarian Cancer.Curr Treat Options Oncol. 2016 Jan;17(1):1. doi: 10.1007/s11864-015-0380-2. Curr Treat Options Oncol. 2016. PMID: 26714493 Review.
-
Primary debulking surgery and neoadjuvant chemotherapy in the treatment of advanced epithelial ovarian carcinoma.Cancer Metastasis Rev. 2015 Mar;34(1):5-10. doi: 10.1007/s10555-014-9536-y. Cancer Metastasis Rev. 2015. PMID: 25597035 Review.
Cited by
-
Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer.Cochrane Database Syst Rev. 2021 Feb 5;2(2):CD005343. doi: 10.1002/14651858.CD005343.pub5. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2021 Jul 30;7:CD005343. doi: 10.1002/14651858.CD005343.pub6. PMID: 33543776 Free PMC article. Updated.
-
The role of neoadjuvant chemotherapy followed by interval debulking surgery in advanced ovarian cancer: a systematic review and meta-analysis of randomized controlled trials and observational studies.Oncotarget. 2017 Dec 27;9(9):8614-8628. doi: 10.18632/oncotarget.23808. eCollection 2018 Feb 2. Oncotarget. 2017. PMID: 29492221 Free PMC article.
-
Neoadjuvant Chemotherapy for Newly Diagnosed, Advanced Ovarian Cancer: ASCO Guideline Update.J Clin Oncol. 2025 Mar;43(7):868-891. doi: 10.1200/JCO-24-02589. Epub 2025 Jan 22. J Clin Oncol. 2025. PMID: 39841949 Free PMC article.
-
Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer.Cochrane Database Syst Rev. 2019 Oct 31;2019(10):CD005343. doi: 10.1002/14651858.CD005343.pub4. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2021 Feb 5;2:CD005343. doi: 10.1002/14651858.CD005343.pub5. PMID: 31684686 Free PMC article. Updated.
-
Neoadjuvant chemotherapy versus primary debulking surgery in advanced epithelial ovarian cancer: A meta-analysis of peri-operative outcome.PLoS One. 2017 Oct 23;12(10):e0186725. doi: 10.1371/journal.pone.0186725. eCollection 2017. PLoS One. 2017. PMID: 29059209 Free PMC article.
References
-
- Kehoe S. et al.. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 386, 249–257 (2015). - PubMed
-
- Vergote I. et al.. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N. Engl. J. Med. 363, 943–953 (2010). - PubMed
-
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: original cancer including fallopian tube cancer and primary peritoneal cancer. v. 2. Avaible at www. nccn. org (2015).
-
- Rose P. G. et al.. Secondary surgical cytoreduction for advanced ovarian carcinoma. N. Engl. J. Med. 351, 2489–2497 (2004). - PubMed
-
- van der Burg M. E. et al.. The effect of debulking surgery after induction chemotherapy on the prognosis in advanced epithelial ovarian cancer. Gynecological Cancer Cooperative Group of the European Organization for Research and Treatment of Cancer. N. Engl. J. Med. 332, 629–634 (1995). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

