miR-19b Regulates Ventricular Action Potential Duration in Zebrafish
- PMID: 27805004
- PMCID: PMC5090966
- DOI: 10.1038/srep36033
miR-19b Regulates Ventricular Action Potential Duration in Zebrafish
Abstract
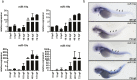
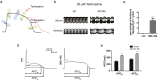
Sudden cardiac death due to ventricular arrhythmias often caused by action potential duration (APD) prolongation is a common mode of death in heart failure (HF). microRNAs, noncoding RNAs that fine tune gene expression, are frequently dysregulated during HF, suggesting a potential involvement in the electrical remodeling process accompanying HF progression. Here, we identified miR-19b as an important regulator of heart function. Zebrafish lacking miR-19b developed severe bradycardia and reduced cardiac contractility. miR-19b deficient fish displayed increased sensitivity to AV-block, a characteristic feature of long QT syndrome in zebrafish. Patch clamp experiments from whole hearts showed that miR-19b deficient zebrafish exhibit significantly prolonged ventricular APD caused by impaired repolarization. We found that miR-19b directly and indirectly regulates the expression of crucial modulatory subunits of cardiac ion channels, and thereby modulates AP duration and shape. Interestingly, miR-19b knockdown mediated APD prolongation can rescue a genetically induced short QT phenotype. Thus, miR-19b might represent a crucial modifier of the cardiac electrical activity, and our work establishes miR-19b as a potential candidate for human long QT syndrome.
Figures




References
-
- Lee J. K. et al. Stage-dependent changes in membrane currents in rats with monocrotaline-induced right ventricular hypertrophy. Am J Physiol. 272, H2833–H2842 (1997). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

