Lumacaftor/Ivacaftor in Patients Aged 6-11 Years with Cystic Fibrosis and Homozygous for F508del-CFTR
- PMID: 27805836
- PMCID: PMC5440888
- DOI: 10.1164/rccm.201608-1754OC
Lumacaftor/Ivacaftor in Patients Aged 6-11 Years with Cystic Fibrosis and Homozygous for F508del-CFTR
Abstract
Rationale: Combination lumacaftor/ivacaftor has been shown to improve lung function and other endpoints in patients aged 12 years and older with cystic fibrosis and homozygous for F508del-CFTR, but it has not been assessed in younger patients.
Objectives: In this open-label phase III trial, we evaluated the safety, tolerability, pharmacodynamics, and efficacy of lumacaftor/ivacaftor combination therapy in patients aged 6-11 years with cystic fibrosis who were homozygous for F508del-CFTR.
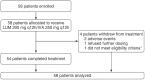
Methods: Patients (N = 58) received 200 mg lumacaftor/250 mg ivacaftor orally every 12 hours for 24 weeks in addition to their existing cystic fibrosis medications.
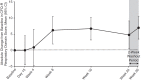
Measurements and main results: Lumacaftor/ivacaftor was well tolerated; the safety profile was generally similar to that observed in larger lumacaftor/ivacaftor trials with older patients. Four patients discontinued (two because of drug-related adverse events: elevated liver transaminases, n = 1; rash, n = 1). No safety concerns were associated with spirometry. No significant changes in percent predicted FEV1 were observed (change from baseline at Week 24, +2.5 percentage points; 95% confidence interval [CI], -0.2 to 5.2; P = 0.0671). At Week 24, significant improvements from baseline were observed in sweat chloride (-24.8 mmol/L; 95% CI, -29.1 to -20.5; P < 0.0001), body mass index z score (+0.15; 95% CI, 0.08 to 0.22; P < 0.0001), Cystic Fibrosis Questionnaire-Revised respiratory domain score (+5.4; 95% CI, 1.4 to 9.4; P = 0.0085), and lung clearance index based on lung volume turnover required to reach 2.5% of starting N2 concentration (-0.88; 95% CI, -1.40 to -0.37; P = 0.0018).
Conclusions: Lumacaftor/ivacaftor was well tolerated in this young population; no new safety concerns were identified. Improvements in lung clearance index, sweat chloride, nutritional status, and health-related quality of life were observed after 24 weeks of treatment. Clinical trial registered with www.clinicaltrials.gov (NCT01897233).
Keywords: cystic fibrosis transmembrane conductance regulator protein; ivacaftor; lumacaftor; lung clearance index; sweat test.
Figures




Comment in
-
Effects of Lumacaftor/Ivacaftor in a Pediatric Cohort Homozygous for F508del-CFTR.Am J Respir Crit Care Med. 2017 Apr 1;195(7):849-850. doi: 10.1164/rccm.201611-2290ED. Am J Respir Crit Care Med. 2017. PMID: 28362199 No abstract available.
References
-
- Clinical and Functional Translation of CFTR (CFTR2)[accessed 2016 July 6]. Available from: http://cftr2.org/index.php
-
- European Cystic Fibrosis Society. Karup, Denmark: European Cystic Fibrosis Society; 2016. ECFSPR Patient Registry: 2013 annual report (version 2)
-
- Cystic Fibrosis Canada. Toronto, ON, Canada: Cystic Fibrosis Canada; 2015. Canadian Cystic Fibrosis Registry: 2013 annual report.
-
- Cystic Fibrosis Foundation. Bethesda, MD: Cystic Fibrosis Foundation; 2015. Cystic Fibrosis Foundation Patient Registry: 2014 annual data report.
-
- VanDevanter DR, Kahle JS, O’Sullivan AK, Sikirica S, Hodgkins PS. Cystic fibrosis in young children: a review of disease manifestation, progression, and response to early treatment. J Cyst Fibros. 2016;15:147–157. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

