The nhTMEM16 Scramblase Is Also a Nonselective Ion Channel
- PMID: 27806273
- PMCID: PMC5103024
- DOI: 10.1016/j.bpj.2016.09.032
The nhTMEM16 Scramblase Is Also a Nonselective Ion Channel
Abstract
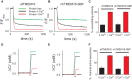
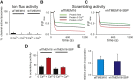
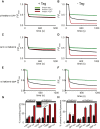
The TMEM16 family comprises Ca2+-activated Cl- channels and phospholipid scramblases. The crystal structure of a fungal homolog, nhTMEM16, revealed an important architectural feature of this protein family in the form of a bilayer-spanning hydrophilic groove that is directly exposed to the membrane. This groove likely provides a pathway for lipid translocation. As mutations that alter ion channel activity of the TMEM16 proteins localize around the groove, it was suggested that the ion and lipid pathways coincide such that the ion pore is partly lined by phospholipids. However, this proposal was not supported by the observation that nhTMEM16 does not mediate ion transport. Here we show that nhTMEM16 mediates both ion and lipid transport and that its properties closely resemble those of a previously characterized fungal homolog, afTMEM16. We show that the reported lack of ion transport activity of nhTMEM16 is due to the lipid composition of the reconstitution membranes and to the presence of a GFP tag. Thus, nhTMEM16, like afTMEM16 and the mammalian TMEM16F, mediates simultaneous lipid scrambling and nonspecific ion transport. This supports the hypothesis that these two processes are tightly correlated and likely to be a general functional feature of the TMEM16 scramblases and therefore of general importance in understanding their biological roles.
Copyright © 2016 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
Comment in
-
TMEM16 Proteins: Membrane Channels with Unusual Pores.Biophys J. 2016 Nov 1;111(9):1821-1822. doi: 10.1016/j.bpj.2016.09.033. Biophys J. 2016. PMID: 27806263 Free PMC article. No abstract available.
References
-
- Caputo A., Caci E., Galietta L.J. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. - PubMed
-
- Yang Y.D., Cho H., Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–1215. - PubMed
-
- Suzuki J., Umeda M., Nagata S. Calcium-dependent phospholipid scrambling by TMEM16F. Nature. 2010;468:834–838. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

