Myosin MgADP Release Rate Decreases as Sarcomere Length Increases in Skinned Rat Soleus Muscle Fibers
- PMID: 27806282
- PMCID: PMC5103013
- DOI: 10.1016/j.bpj.2016.09.024
Myosin MgADP Release Rate Decreases as Sarcomere Length Increases in Skinned Rat Soleus Muscle Fibers
Abstract
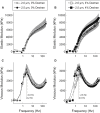
Actin-myosin cross-bridges use chemical energy from MgATP hydrolysis to generate force and shortening in striated muscle. Previous studies show that increases in sarcomere length can reduce thick-to-thin filament spacing in skinned muscle fibers, thereby increasing force production at longer sarcomere lengths. However, it is unclear how changes in sarcomere length and lattice spacing affect cross-bridge kinetics at fundamental steps of the cross-bridge cycle, such as the MgADP release rate. We hypothesize that decreased lattice spacing, achieved through increased sarcomere length or osmotic compression of the fiber via dextran T-500, could slow MgADP release rate and increase cross-bridge attachment duration. To test this, we measured cross-bridge cycling and MgADP release rates in skinned soleus fibers using stochastic length-perturbation analysis at 2.5 and 2.0 μm sarcomere lengths as pCa and [MgATP] varied. In the absence of dextran, the force-pCa relationship showed greater Ca2+ sensitivity for 2.5 vs. 2.0 μm sarcomere length fibers (pCa50 = 5.68 ± 0.01 vs. 5.60 ± 0.01). When fibers were compressed with 4% dextran, the length-dependent increase in Ca2+ sensitivity of force was attenuated, though the Ca2+ sensitivity of the force-pCa relationship at both sarcomere lengths was greater with osmotic compression via 4% dextran compared to no osmotic compression. Without dextran, the cross-bridge detachment rate slowed by ∼15% as sarcomere length increased, due to a slower MgADP release rate (11.2 ± 0.5 vs. 13.5 ± 0.7 s-1). In the presence of dextran, cross-bridge detachment was ∼20% slower at 2.5 vs. 2.0 μm sarcomere length due to a slower MgADP release rate (10.1 ± 0.6 vs. 12.9 ± 0.5 s-1). However, osmotic compression of fibers at either 2.5 or 2.0 μm sarcomere length produced only slight (and statistically insignificant) slowing in the rate of MgADP release. These data suggest that skeletal muscle exhibits sarcomere-length-dependent changes in cross-bridge kinetics and MgADP release that are separate from, or complementary to, changes in lattice spacing.
Copyright © 2016 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Konhilas J.P., Irving T.C., de Tombe P.P. Frank-Starling law of the heart and the cellular mechanisms of length-dependent activation. Pflugers Arch. 2002;445:305–310. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

