High-fat diet-induced glucose dysregulation is independent of changes in islet ACE2 in mice
- PMID: 27806985
- PMCID: PMC5256982
- DOI: 10.1152/ajpregu.00362.2016
High-fat diet-induced glucose dysregulation is independent of changes in islet ACE2 in mice
Abstract
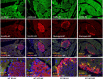
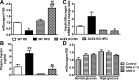
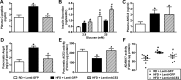
While restoration of ACE2 activity in the pancreas leads to improvement of glycemia in experimental models of Type 2 diabetes, global deficiency in ACE2 disrupts β-cell function and impairs glucose tolerance in mice, demonstrating the physiological role of ACE2 in glucose homeostasis. Although the contribution of pancreatic ACE2 to glucose regulation has been demonstrated in genetic models of diabetes and in models with overexpression of the renin-angiotensin system (RAS), it is unclear whether islet ACE2 is involved in glycemic control in common models of human Type 2 diabetes. To determine whether diet-induced diabetes deregulates glucose homeostasis via reduction of ACE2 in the pancreatic islets, wild-type (WT) and ACE2 knockout (KO) male mice were fed a high-fat diet (HFD) for 16 wk. ACE2 KO mice were more susceptible than WT mice to HFD-mediated glycemic dysregulation. Islet ACE2 activity and expression of various genes, including ANG II type 1a receptor (mAT1aR) were then assessed. Surprisingly, we observed no change in islet ACE2 activity and expression despite local RAS overactivity, indicated by an upregulation of mAT1aR expression. Despite a predominant expression in islet α-cells, further investigation highlighted a minor role for ACE2 on glucagon expression. Further, pancreatic ACE2 gene therapy improved glycemia in HFD-fed WT mice, leading to enhanced glucose-stimulated insulin secretion, reduced pancreatic ANG II levels, fibrosis, and ADAM17 activity. Altogether, our study demonstrates that HFD feeding increases RAS activity and mediates glycemic dysregulation likely through loss of ACE2 present outside the islets but independently of changes in islet ACE2.
Keywords: ACE2; Type 2 diabetes; high-fat diet; pancreatic islets; renin-angiotensin system.
Copyright © 2016 the American Physiological Society.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

