Fibroblast Growth Requires CT10 Regulator of Kinase (Crk) and Crk-like (CrkL)
- PMID: 27807028
- PMCID: PMC5159491
- DOI: 10.1074/jbc.M116.764613
Fibroblast Growth Requires CT10 Regulator of Kinase (Crk) and Crk-like (CrkL)
Abstract
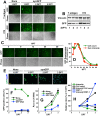
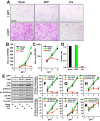
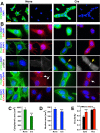
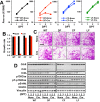
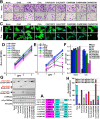
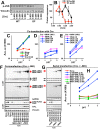
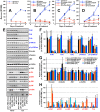
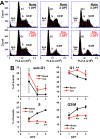
CT10 regulator of kinase (Crk) and Crk-like (CrkL) are the cellular counterparts of the viral oncogene v-Crk Elevated levels of Crk and CrkL have been observed in many human cancers; inhibition of Crk and CrkL expression reduced the tumor-forming potential of cancer cell lines. Despite a close relationship between the Crk family proteins and tumorigenesis, how Crk and CrkL contribute to cell growth is unclear. We ablated endogenous Crk and CrkL from cultured fibroblasts carrying floxed alleles of Crk and CrkL by transfection with synthetic Cre mRNA (synCre). Loss of Crk and CrkL induced by synCre transfection blocked cell proliferation and caused shrinkage of the cytoplasm and the nucleus, formation of adherens junctions, and reduced cell motility. Ablation of Crk or CrkL alone conferred a much more modest reduction in cell proliferation. Reintroduction of CrkI, CrkII, or CrkL individually rescued cell proliferation in the absence of the endogenous Crk and CrkL, suggesting that Crk and CrkL play overlapping functions in regulating fibroblast growth. Serum and basic FGF induced phosphorylation of Akt, MAP kinases, and S6 kinase and Fos expression in the absence of Crk and CrkL, suggesting that cells lacking Crk and CrkL are capable of initiating major signal transduction pathways in response to extracellular stimuli. Furthermore, cell cycle and cell death analyses demonstrated that fibroblasts lacking Crk and CrkL become arrested at the G1-S transition and undergo a modest apoptosis. Taken together, our results suggest that Crk and CrkL play essential overlapping roles in fibroblast growth.
Keywords: adaptor protein; apoptosis; cell cycle; cell growth; fibroblast; growth factor; serum.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Mayer B. J., Hamaguchi M., and Hanafusa H. (1988) Characterization of p47gag-crk, a novel oncogene product with sequence similarity to a putative modulatory domain of protein-tyrosine kinases and phospholipase C. Cold Spring Harb. Symp. Quant. Biol. 53, 907–914 - PubMed
-
- Kumar S., Fajardo J. E., Birge R. B., and Sriram G. (2014) Crk at the quarter century mark: perspectives in signaling and cancer. J. Cell. Biochem. 115, 819–825 - PubMed
-
- Senechal K., Halpern J., and Sawyers C. L. (1996) The CRKL adaptor protein transforms fibroblasts and functions in transformation by the BCR-ABL oncogene. J. Biol. Chem. 271, 23255–23261 - PubMed
-
- Yamada S., Yanamoto S., Kawasaki G., Rokutanda S., Yonezawa H., Kawakita A., and Nemoto T. K. (2011) Overexpression of CRKII increases migration and invasive potential in oral squamous cell carcinoma. Cancer Lett. 303, 84–91 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

