Cellular Nuclear Export Factors TAP and Aly Are Required for HDAg-L-mediated Assembly of Hepatitis Delta Virus
- PMID: 27807029
- PMCID: PMC5207089
- DOI: 10.1074/jbc.M116.754853
Cellular Nuclear Export Factors TAP and Aly Are Required for HDAg-L-mediated Assembly of Hepatitis Delta Virus
Abstract
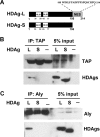
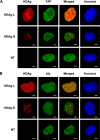
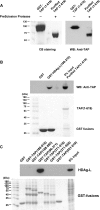
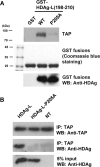
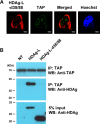
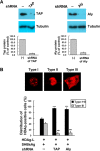
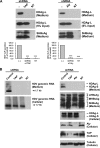
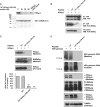
Hepatitis delta virus (HDV) is a satellite virus of hepatitis B virus (HBV). HDV genome encodes two forms of hepatitis delta antigen (HDAg), small HDAg (HDAg-S), which is required for viral replication, and large HDAg (HDAg-L), which is essential for viral assembly. HDAg-L is identical to HDAg-S except that it bears a 19-amino acid extension at the C terminus. Both HDAgs contain a nuclear localization signal (NLS), but only HDAg-L contains a CRM1-independent nuclear export signal at its C terminus. The nuclear export activity of HDAg-L is important for HDV particle formation. However, the mechanisms of HDAg-L-mediated nuclear export of HDV ribonucleoprotein are not clear. In this study, the host cellular RNA export complex TAP-Aly was found to form a complex with HDAg-L, but not with an export-defective HDAg-L mutant, in which Pro205 was replaced by Ala. HDAg-L was found to colocalize with TAP and Aly in the nucleus. The C-terminal domain of HDAg-L was shown to directly interact with the N terminus of TAP, whereas an HDAg-L mutant lacking the NLS failed to interact with full-length TAP. In addition, small hairpin RNA-mediated down-regulation of TAP or Aly reduced nuclear export of HDAg-L and assembly of HDV virions. Furthermore, a peptide, TAT-HDAg-L(198-210), containing the 10-amino acid TAT peptide and HDAg-L(198-210), inhibited the interaction between HDAg-L and TAP and blocked HDV virion assembly and secretion. These data demonstrate that formation and release of HDV particles are mediated by TAP and Aly.
Keywords: Aly; TAP; hepatitis delta antigen; hepatitis virus; nuclear export factor; nuclear transport; protein translocation; viral replication; virus assembly.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
Similar articles
-
Tat-enhanced delivery of the C terminus of HDAg-L inhibits assembly and secretion of hepatitis D virus.Antiviral Res. 2018 Feb;150:69-78. doi: 10.1016/j.antiviral.2017.12.009. Epub 2017 Dec 14. Antiviral Res. 2018. PMID: 29247673
-
Casein kinase II and protein kinase C modulate hepatitis delta virus RNA replication but not empty viral particle assembly.J Virol. 1996 Sep;70(9):6190-8. doi: 10.1128/JVI.70.9.6190-6198.1996. J Virol. 1996. PMID: 8709245 Free PMC article.
-
Clathrin-mediated post-Golgi membrane trafficking in the morphogenesis of hepatitis delta virus.J Virol. 2009 Dec;83(23):12314-24. doi: 10.1128/JVI.01044-09. Epub 2009 Sep 30. J Virol. 2009. PMID: 19793827 Free PMC article.
-
Future treatments for hepatitis delta virus infection.Liver Int. 2020 Feb;40 Suppl 1:54-60. doi: 10.1111/liv.14356. Liver Int. 2020. PMID: 32077603 Review.
-
An update on HDV: virology, pathogenesis and treatment.Antivir Ther. 2013;18(3 Pt B):541-8. doi: 10.3851/IMP2598. Epub 2013 Jun 21. Antivir Ther. 2013. PMID: 23792471 Review.
Cited by
-
Innate immune recognition and modulation in hepatitis D virus infection.World J Gastroenterol. 2020 Jun 7;26(21):2781-2791. doi: 10.3748/wjg.v26.i21.2781. World J Gastroenterol. 2020. PMID: 32550754 Free PMC article. Review.
-
Hepatitis B virus entry, assembly, and egress.Microbiol Mol Biol Rev. 2024 Dec 18;88(4):e0001424. doi: 10.1128/mmbr.00014-24. Epub 2024 Oct 23. Microbiol Mol Biol Rev. 2024. PMID: 39440957 Review.
-
Targeting the Host for New Therapeutic Perspectives in Hepatitis D.J Clin Med. 2020 Jan 14;9(1):222. doi: 10.3390/jcm9010222. J Clin Med. 2020. PMID: 31947588 Free PMC article. Review.
-
Hepatitis Delta Virus: Replication Strategy and Upcoming Therapeutic Options for a Neglected Human Pathogen.Viruses. 2017 Jul 4;9(7):172. doi: 10.3390/v9070172. Viruses. 2017. PMID: 28677645 Free PMC article. Review.
-
Cellular Factors Involved in the Hepatitis D Virus Life Cycle.Viruses. 2023 Aug 3;15(8):1687. doi: 10.3390/v15081687. Viruses. 2023. PMID: 37632029 Free PMC article. Review.
References
-
- Rizzetto M. (1983) The delta agent. Hepatology 3, 729–737 - PubMed
-
- Lai M. M. (1995) The molecular biology of hepatitis delta virus. Annu. Rev. Biochem. 64, 259–286 - PubMed
-
- Weiner A. J., Choo Q. L., Wang K. S., Govindarajan S., Redeker A. G., Gerin J. L., and Houghton M. (1988) A single antigenomic open reading frame of the hepatitis delta virus encodes the epitope(s) of both hepatitis delta antigen polypeptides p24 delta and p27 delta. J. Virol. 62, 594–599 - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

