HIF-KDM3A-MMP12 regulatory circuit ensures trophoblast plasticity and placental adaptations to hypoxia
- PMID: 27807143
- PMCID: PMC5135375
- DOI: 10.1073/pnas.1612626113
HIF-KDM3A-MMP12 regulatory circuit ensures trophoblast plasticity and placental adaptations to hypoxia
Abstract
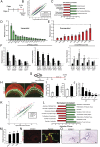
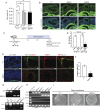
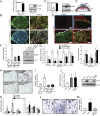
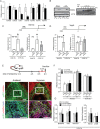
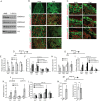
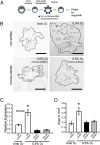
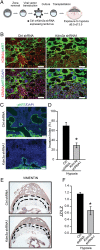
The hemochorial placenta develops from the coordinated multilineage differentiation of trophoblast stem (TS) cells. An invasive trophoblast cell lineage remodels uterine spiral arteries, facilitating nutrient flow, failure of which is associated with pathological conditions such as preeclampsia, intrauterine growth restriction, and preterm birth. Hypoxia plays an instructive role in influencing trophoblast cell differentiation and regulating placental organization. Key downstream hypoxia-activated events were delineated using rat TS cells and tested in vivo, using trophoblast-specific lentiviral gene delivery and genome editing. DNA microarray analyses performed on rat TS cells exposed to ambient or low oxygen and pregnant rats exposed to ambient or hypoxic conditions showed up-regulation of genes characteristic of an invasive/vascular remodeling/inflammatory phenotype. Among the shared up-regulated genes was matrix metallopeptidase 12 (MMP12). To explore the functional importance of MMP12 in trophoblast cell-directed spiral artery remodeling, we generated an Mmp12 mutant rat model using transcription activator-like nucleases-mediated genome editing. Homozygous mutant placentation sites showed decreased hypoxia-dependent endovascular trophoblast invasion and impaired trophoblast-directed spiral artery remodeling. A link was established between hypoxia/HIF and MMP12; however, evidence did not support Mmp12 as a direct target of HIF action. Lysine demethylase 3A (KDM3A) was identified as mediator of hypoxia/HIF regulation of Mmp12 Knockdown of KDM3A in rat TS cells inhibited the expression of a subset of the hypoxia-hypoxia inducible factor (HIF)-dependent transcripts, including Mmp12, altered H3K9 methylation status, and decreased hypoxia-induced trophoblast cell invasion in vitro and in vivo. The hypoxia-HIF-KDM3A-MMP12 regulatory circuit is conserved and facilitates placental adaptations to environmental challenges.
Keywords: epigenetics; hypoxia; placenta; plasticity; trophoblast invasion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Georgiades P, Ferguson-Smith AC, Burton GJ. Comparative developmental anatomy of the murine and human definitive placentae. Placenta. 2002;23(1):3–19. - PubMed
-
- Harris LK. Review: Trophoblast-vascular cell interactions in early pregnancy: How to remodel a vessel. Placenta. 2010;31(Suppl):S93–S98. - PubMed
-
- Maltepe E, Fisher SJ. Placenta: The forgotten organ. Annu Rev Cell Dev Biol. 2015;31:523–552. - PubMed
-
- Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003;69(1):1–7. - PubMed
-
- Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: Facts and controversies. Placenta. 2006;27(9-10):939–958. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

