Tacrolimus Monotherapy after Intravenous Methylprednisolone in Adults with Minimal Change Nephrotic Syndrome
- PMID: 27807213
- PMCID: PMC5373446
- DOI: 10.1681/ASN.2016030342
Tacrolimus Monotherapy after Intravenous Methylprednisolone in Adults with Minimal Change Nephrotic Syndrome
Abstract
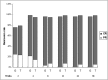
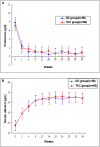
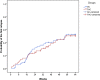
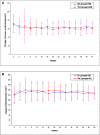
Glucocorticoid treatment is the first choice therapy for adults with minimal change nephrotic syndrome; however, this therapy associates with many adverse effects. Tacrolimus may be an alternative to conventional glucocorticoid therapy. To investigate this possibility, we conducted a prospective, randomized, controlled trial (WHO International Clinical Trials Registry Platform: ChiCTR-TRC-11001454) in eight renal units across China. We randomized enrolled patients with adult-onset minimal change nephrotic syndrome (n=119) to receive glucocorticoid therapy or tacrolimus after intravenous methylprednisolone (0.8 mg/kg per day) for 10 days. Patients received a conventional glucocorticoid regimen or tacrolimus monotherapy, starting with 0.05 mg/kg per day (target trough whole-blood level of 4-8 ng/ml) for 16-20 weeks and subsequently tapering over approximately 18 weeks. Remission occurred in 51 of 53 (96.2%; all complete remission) glucocorticoid-treated patients and 55 of 56 (98.3%; 52 complete and three partial remission) tacrolimus-treated patients (P=0.61 for remission; P=0.68 for complete remission). The groups had similar mean time to remission (P=0.55). Relapse occurred in 49.0% and 45.5% of the glucocorticoid- and tacrolimus-treated patients, respectively (P=0.71), with similar time to relapse (P=0.86). Seven (13.7%) glucocorticoid-treated and four (7.3%) tacrolimus-treated patients suffered frequent relapse (P=0.28); five glucocorticoid-treated and two tacrolimus-treated patients became drug dependent (P=0.26). Adverse events occurred more frequently in the glucocorticoid group (128 versus 81 in the tacrolimus group). Seven adverse events in the glucocorticoid group and two adverse events in the tacrolimus group were serious. Consequently, tacrolimus monotherapy after short-term intravenous methylprednisolone is noninferior to conventional glucocorticoid treatment for adult-onset minimal change nephrotic syndrome in this cohort.
Keywords: adults; clinical trial; minimal change nephrotic syndrome; monotherapy; tacrolimus.
Copyright © 2017 by the American Society of Nephrology.
Figures
References
-
- Bargman JM: Management of minimal lesion glomerulonephritis: Evidence-based recommendations. Kidney Int Suppl 70: S3–S16, 1999 - PubMed
-
- Hogan J, Radhakrishnan J: The treatment of minimal change disease in adults. J Am Soc Nephrol 24: 702–711, 2013 - PubMed
-
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephrits Work Group : KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl 2: 139–274, 2012
-
- Szeto CC, Lai FM, Chow KM, Kwan BC, Kwong VW, Leung CB, Li PK: Long-term outcome of biopsy-proven minimal change nephropathy in Chinese adults. Am J Kidney Dis 65: 710–718, 2015 - PubMed
-
- Waldman M, Crew RJ, Valeri A, Busch J, Stokes B, Markowitz G, D’Agati V, Appel G: Adult minimal-change disease: Clinical characteristics, treatment, and outcomes. Clin J Am Soc Nephrol 2: 445–453, 2007 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

