Top-down models in biology: explanation and control of complex living systems above the molecular level
- PMID: 27807271
- PMCID: PMC5134011
- DOI: 10.1098/rsif.2016.0555
Top-down models in biology: explanation and control of complex living systems above the molecular level
Abstract
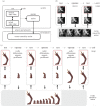
It is widely assumed in developmental biology and bioengineering that optimal understanding and control of complex living systems follows from models of molecular events. The success of reductionism has overshadowed attempts at top-down models and control policies in biological systems. However, other fields, including physics, engineering and neuroscience, have successfully used the explanations and models at higher levels of organization, including least-action principles in physics and control-theoretic models in computational neuroscience. Exploiting the dynamic regulation of pattern formation in embryogenesis and regeneration requires new approaches to understand how cells cooperate towards large-scale anatomical goal states. Here, we argue that top-down models of pattern homeostasis serve as proof of principle for extending the current paradigm beyond emergence and molecule-level rules. We define top-down control in a biological context, discuss the examples of how cognitive neuroscience and physics exploit these strategies, and illustrate areas in which they may offer significant advantages as complements to the mainstream paradigm. By targeting system controls at multiple levels of organization and demystifying goal-directed (cybernetic) processes, top-down strategies represent a roadmap for using the deep insights of other fields for transformative advances in regenerative medicine and systems bioengineering.
Keywords: cognitive modelling; developmental biology; integrative; regeneration; remodelling; top-down.
© 2016 The Author(s).
Figures



References
-
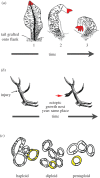
- Farinella-Ferruzza N. 1956. The transformation of a tail into limb after xenoplastic transplantation. Experientia 12, 304–305. ( 10.1007/BF02159624) - DOI
-
- Mustard J, Levin M. 2014. Bioelectrical mechanisms for programming growth and form: taming physiological networks for soft body robotics. Soft Robot. 1, 169–191. ( 10.1089/soro.2014.0011) - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
