Cancer and Chemotherapy Contribute to Muscle Loss by Activating Common Signaling Pathways
- PMID: 27807421
- PMCID: PMC5070123
- DOI: 10.3389/fphys.2016.00472
Cancer and Chemotherapy Contribute to Muscle Loss by Activating Common Signaling Pathways
Abstract
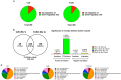
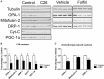
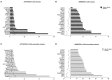
Cachexia represents one of the primary complications of colorectal cancer due to its effects on depletion of muscle and fat. Evidence suggests that chemotherapeutic regimens, such as Folfiri, contribute to cachexia-related symptoms. The purpose of the present study was to investigate the cachexia signature in different conditions associated with severe muscle wasting, namely Colon-26 (C26) and Folfiri-associated cachexia. Using a quantitative LC-MS/MS approach, we identified significant changes in 386 proteins in the quadriceps muscle of Folfiri-treated mice, and 269 proteins differentially expressed in the C26 hosts (p < 0.05; -1.5 ≥ fold change ≥ +1.5). Comparative analysis isolated 240 proteins that were modulated in common, with a large majority (218) that were down-regulated in both experimental settings. Interestingly, metabolic (47.08%) and structural (21.25%) proteins were the most represented. Pathway analysis revealed mitochondrial dysfunctions in both experimental conditions, also consistent with reduced expression of mediators of mitochondrial fusion (OPA-1, mitofusin-2), fission (DRP-1) and biogenesis (Cytochrome C, PGC-1α). Alterations of oxidative phosphorylation within the TCA cycle, fatty acid metabolism, and Ca2+ signaling were also detected. Overall, the proteomic signature in the presence of both chemotherapy and cancer suggests the activation of mechanisms associated with movement disorders, necrosis, muscle cell death, muscle weakness and muscle damage. Conversely, this is consistent with the inhibition of pathways that regulate nucleotide and fatty acid metabolism, synthesis of ATP, muscle and heart function, as well as ROS scavenging. Interestingly, strong up-regulation of pro-inflammatory acute-phase proteins and a more coordinated modulation of mitochondrial and lipidic metabolisms were observed in the muscle of the C26 hosts that were different from the Folfiri-treated animals. In conclusion, our results suggest that both cancer and chemotherapy contribute to muscle loss by activating common signaling pathways. These data support the undertaking of combination strategies that aim to both counteract tumor growth and reduce chemotherapy side effects.
Keywords: C26; Folfiri; cachexia; inflammation; mitochondria; mitochondrial fusion and fission; muscle; proteomics.
Figures



References
-
- American Cancer Society (2015). Cancer Statistics. Atlanta, GE: American Cancer Society.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

