Tetrapyrrole Signaling in Plants
- PMID: 27807442
- PMCID: PMC5069423
- DOI: 10.3389/fpls.2016.01586
Tetrapyrrole Signaling in Plants
Abstract
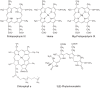
Tetrapyrroles make critical contributions to a number of important processes in diverse organisms. In plants, tetrapyrroles are essential for light signaling, the detoxification of reactive oxygen species, the assimilation of nitrate and sulfate, respiration, photosynthesis, and programed cell death. The misregulation of tetrapyrrole metabolism can produce toxic reactive oxygen species. Thus, it is not surprising that tetrapyrrole metabolism is strictly regulated and that tetrapyrrole metabolism affects signaling mechanisms that regulate gene expression. In plants and algae, tetrapyrroles are synthesized in plastids and were some of the first plastid signals demonstrated to regulate nuclear gene expression. In plants, the mechanism of tetrapyrrole-dependent plastid-to-nucleus signaling remains poorly understood. Additionally, some of experiments that tested ideas for possible signaling mechanisms appeared to produce conflicting data. In some instances, these conflicts are potentially explained by different experimental conditions. Although the biological function of tetrapyrrole signaling is poorly understood, there is compelling evidence that this signaling is significant. Specifically, this signaling appears to affect the accumulation of starch and may promote abiotic stress tolerance. Tetrapyrrole-dependent plastid-to-nucleus signaling interacts with a distinct plastid-to-nucleus signaling mechanism that depends on GENOMES UNCUOPLED1 (GUN1). GUN1 contributes to a variety of processes, such as chloroplast biogenesis, the circadian rhythm, abiotic stress tolerance, and development. Thus, the contribution of tetrapyrrole signaling to plant function is potentially broader than we currently appreciate. In this review, I discuss these aspects of tetrapyrrole signaling.
Keywords: Mg-protoporphyrin IX; chlorophyll; chloroplast; heme; plastid; plastid signaling; porphyrin; tetrapyrrole.
Figures


References
-
- Aarti D. P., Tanaka R., Tanaka A. (2006). Effects of oxidative stress on chlorophyll biosynthesis in cucumber (Cucumis sativus) cotyledons. Physiol. Plant. 128 186–197. 10.1111/j.1399-3054.2006.00720.x - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

