Lipopolysaccharide Induces Human Pulmonary Micro-Vascular Endothelial Apoptosis via the YAP Signaling Pathway
- PMID: 27807512
- PMCID: PMC5069405
- DOI: 10.3389/fcimb.2016.00133
Lipopolysaccharide Induces Human Pulmonary Micro-Vascular Endothelial Apoptosis via the YAP Signaling Pathway
Abstract
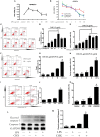
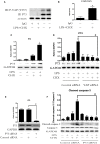
Gram-negative bacterial lipopolysaccharide (LPS) induces a pathologic increase in lung vascular leakage under septic conditions. LPS-induced human pulmonary micro-vascular endothelial cell (HPMEC) apoptosis launches and aggravates micro-vascular hyper-permeability and acute lung injury (ALI). Previous studies show that the activation of intrinsic apoptotic pathway is vital for LPS-induced EC apoptosis. Yes-associated protein (YAP) has been reported to positively regulate intrinsic apoptotic pathway in tumor cells apoptosis. However, the potential role of YAP protein in LPS-induced HPMEC apoptosis has not been determined. In this study, we found that LPS-induced activation and nuclear accumulation of YAP accelerated HPMECs apoptosis. LPS-induced YAP translocation from cytoplasm to nucleus by the increased phosphorylation on Y357 resulted in the interaction between YAP and transcription factor P73. Furthermore, inhibition of YAP by small interfering RNA (siRNA) not only suppressed the LPS-induced HPMEC apoptosis but also regulated P73-mediated up-regulation of BAX and down-regulation of BCL-2. Taken together, our results demonstrated that activation of the YAP/P73/(BAX and BCL-2)/caspase-3 signaling pathway played a critical role in LPS-induced HPMEC apoptosis. Inhibition of the YAP might be a potential therapeutic strategy for lung injury under sepsis.
Keywords: P73; Yes-associated protein; apoptosis; endothelial cells; lipopolysaccharide.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

