Learning to experience side effects after antidepressant intake - Results from a randomized, controlled, double-blind study
- PMID: 27807605
- PMCID: PMC5225191
- DOI: 10.1007/s00213-016-4466-8
Learning to experience side effects after antidepressant intake - Results from a randomized, controlled, double-blind study
Abstract
Background: Side effects play a key role in patients' failure to take antidepressants. There is evidence that verbal suggestions and informed consent elicit expectations that can in turn trigger the occurrence of side effects. Prior experience or learning mechanisms are also assumed to contribute to the development of side effects, although their role has not been thoroughly investigated. In this study, we examined whether an antidepressant's side effects can be learned via Pavlovian conditioning.
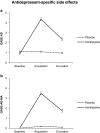
Methods: Participants (n = 39) were randomly allocated to one of two groups and were exposed to a classical conditioning procedure. During acquisition, 19 participants received amitriptyline and 20 participants received a placebo pill. Pills were taken for four nights together with a novel-tasting drink. After a washout phase, both groups received a placebo pill together with the novel-tasting drink (evocation). Side effects were assessed via the Generic Assessment of Side Effects Scale prior to acquisition (baseline), after acquisition, and after evocation. A score of antidepressant-specific side effects was calculated.
Results: Participants taking amitriptyline reported significantly more antidepressant-specific side effects after acquisition compared to both baseline and the placebo group. After evocation, participants who underwent the conditioning procedure with amitriptyline reported significantly more antidepressant-specific side effects than those who never received amitriptyline, even though both groups received a placebo.
Conclusions: Our results indicate that antidepressant side effects can be learned using a conditioning paradigm and evoked via a placebo pill when applied with the same contextual factors as the verum.
Keywords: Antidepressants; Learning; Nocebo; Side effects.
Conflict of interest statement
Compliance with ethical standards Funding The study was prepared in the context of the FOR1328 research unit on placebo and nocebo mechanisms and was partially supported by a grant from the German Research Foundation (Deutsche Forschungsgemeinschaft (DFG), RI 574/22–1). Conflict of interest The authors Julia Rheker, Alexander Winkler, Bettina K. Doering, and Winfried Rief have no conflicts of interest including any financial, personal, or other relationships with other people or organizations to declare that could inappropriately influence, or be perceived to influence, the present work. However, Bettina K. Doering has received honoraria from Biologische Heilmittel Heel for presentations on stress reactions. Winfried Rief has received honoraria from Biologische Heilmittel Heel, Berlin Chemie, and Bayer for presentations on placebo effects.
Figures


References
-
- Abbing-Karahagopian V, Huerta C, Souverein PC, et al. Antidepressant prescribing in five European countries: application of common definitions to assess the prevalence, clinical observations, and methodological implications. Eur J Clin Pharmacol. 2014;70:849–857. doi: 10.1007/s00228-014-1676-z. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

