Homoserine as an Aspartic Acid Precursor for Synthesis of Proteoglycan Glycopeptide Containing Aspartic Acid and a Sulfated Glycan Chain
- PMID: 27809505
- PMCID: PMC5215661
- DOI: 10.1021/acs.joc.6b02441
Homoserine as an Aspartic Acid Precursor for Synthesis of Proteoglycan Glycopeptide Containing Aspartic Acid and a Sulfated Glycan Chain
Abstract
Among many hurdles in synthesizing proteoglycan glycopeptides, one challenge is the incorporation of aspartic acid in the peptide backbone and acid sensitive O-sulfated glycan chains. To overcome this, a new strategy was developed utilizing homoserine as an aspartic acid precursor. The conversion of homoserine to aspartic acid in the glycopeptide was successfully accomplished by late stage oxidation using (2,2,6,6-tetramethyl-piperidin-1-yl)oxyl (TEMPO) and bis(acetoxy)iodobenzene (BAIB). This is the first time that a glycopeptide containing aspartic acid and an O-sulfated glycan was synthesized.
Conflict of interest statement
Notes: The authors declare no competing financial interest.
Figures
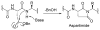

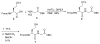


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

