Teicoplanin-based antimicrobial therapy in Staphylococcus aureus bone and joint infection: tolerance, efficacy and experience with subcutaneous administration
- PMID: 27809799
- PMCID: PMC5093939
- DOI: 10.1186/s12879-016-1955-7
Teicoplanin-based antimicrobial therapy in Staphylococcus aureus bone and joint infection: tolerance, efficacy and experience with subcutaneous administration
Abstract
Background: Staphylococci represent the first etiologic agents of bone and joint infection (BJI), leading glycopeptides use, especially in case of methicillin-resistance or betalactam intolerance. Teicoplanin may represent an alternative to vancomycin because of its acceptable bone penetration and possible subcutaneous administration.
Methods: Adults receiving teicoplanin for S. aureus BJI were included in a retrospective cohort study investigating intravenous or subcutaneous teicoplanin safety and pharmacokinetics.
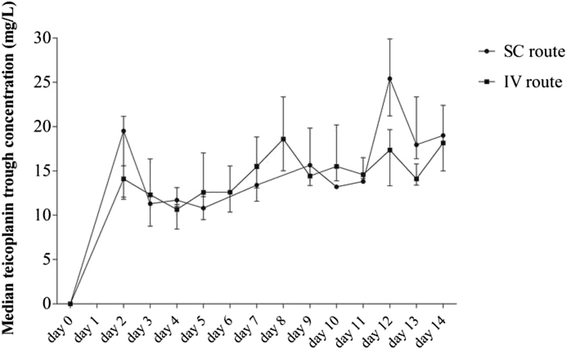
Results: Sixty-five S. aureus BJIs (orthopedic device-related infections, 69 %; methicillin-resistance, 17 %) were treated by teicoplanin at the initial dose of 5.7 mg/kg/day (IQR, 4.7-6.5) after a loading dose of 5 injections 12 h apart. The first trough teicoplanin level (Cmin) reached the therapeutic target (15 mg/L) in 26 % of patients, only. An overdose (Cmin >25 mg/L) was observed in 16 % patients, 50 % of which had chronic renal failure (p = 0.049). Seven adverse events occurred in 6 patients (10 %); no predictive factor could be highlighted. After a 91-week follow-up (IQR, 51-183), 27 treatment failures were observed (42 %), associated with diabetes (OR, 5.1; p = 0.057), systemic inflammatory disease (OR, 5.6; p = 0.043), and abscess (OR, 4.1; p < 10-3). A normal CRP-value at 1 month was protective (OR, 0.2; p = 0.029). Subcutaneous administration (n = 14) showed no difference in pharmacokinetics and tolerance compared to the intravenous route.
Conclusions: Teicoplanin constitutes a well-tolerated therapeutic alternative in S. aureus BJI, with a possible subcutaneous administration in outpatients. The loading dose might be increase to 9-12 mg/kg to quickly reach the therapeutic target, but tolerance of such higher doses remains to be evaluated, especially if using the subcutaneous route.
Keywords: Bone and joint infection; Staphylococcus aureus; Subcutanous administration; Teicoplanin.
Figures
References
-
- Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:e1–25. doi: 10.1093/cid/cis803. - DOI - PubMed
-
- Valour F, Karsenty J, Bouaziz A, Ader F, Tod M, Lustig S, Laurent F, Ecochard R, Chidiac C, Ferry T. Antimicrobial-related severe adverse events during treatment of bone and joint infection due to methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother. 2014;58:746–55. doi: 10.1128/AAC.02032-13. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous