Insight of brain degenerative protein modifications in the pathology of neurodegeneration and dementia by proteomic profiling
- PMID: 27809929
- PMCID: PMC5094070
- DOI: 10.1186/s13041-016-0272-9
Insight of brain degenerative protein modifications in the pathology of neurodegeneration and dementia by proteomic profiling
Abstract
Dementia is a syndrome associated with a wide range of clinical features including progressive cognitive decline and patient inability to self-care. Due to rapidly increasing prevalence in aging society, dementia now confers a major economic, social, and healthcare burden throughout the world, and has therefore been identified as a public health priority by the World Health Organization. Previous studies have established dementia as a 'proteinopathy' caused by detrimental changes in brain protein structure and function that promote misfolding, aggregation, and deposition as insoluble amyloid plaques. Despite clear evidence that pathological cognitive decline is associated with degenerative protein modifications (DPMs) arising from spontaneous chemical modifications to amino acid side chains, the molecular mechanisms that promote brain DPMs formation remain poorly understood. However, the technical challenges associated with DPM analysis have recently become tractable due to powerful new proteomic techniques that facilitate detailed analysis of brain tissue damage over time. Recent studies have identified that neurodegenerative diseases are associated with the dysregulation of critical repair enzymes, as well as the misfolding, aggregation and accumulation of modified brain proteins. Future studies will further elucidate the mechanisms underlying dementia pathogenesis via the quantitative profiling of the human brain proteome and associated DPMs in distinct phases and subtypes of disease. This review summarizes recent developments in quantitative proteomic technologies, describes how these techniques have been applied to the study of dementia-linked changes in brain protein structure and function, and briefly outlines how these findings might be translated into novel clinical applications for dementia patients. In this review, only spontaneous protein modifications such as deamidation, oxidation, nitration glycation and carbamylation are reviewed and discussed.
Keywords: Alzheimer disease; Biomarkers; Deamidation; Degenerative protein modifications (DPMs); Dementia; Neurodegenerative disease; Nitration; Oxidation; β-amyloid.
Figures
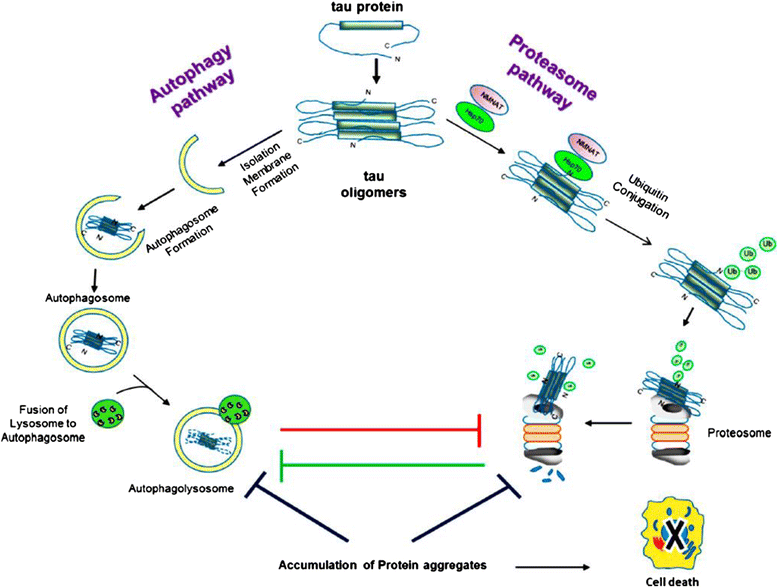

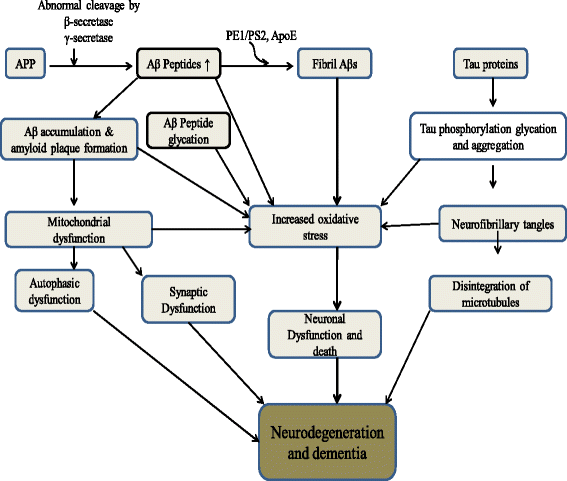
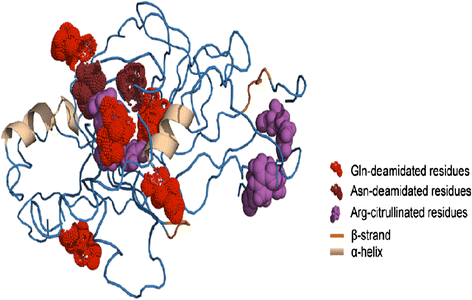
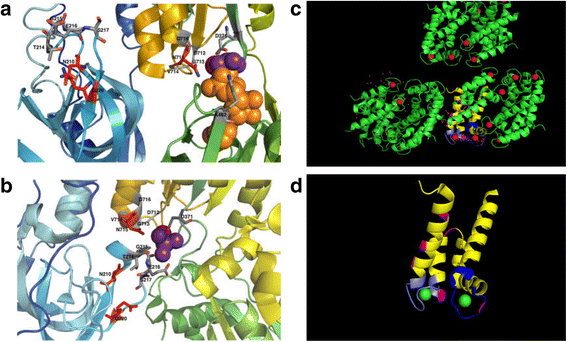
References
-
- WHO . Dementia. 2016.
-
- WHO . Dementia: a public health priority, dementia report. 2012.
-
- Editorial. Great expectations for dementia research. Lancet Neurol. 15(2):125. http://thelancet.com/journals/laneur/article/PIIS1474-4422(15)00394-4/fu.... - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

