Embryonic exposures to perfluorooctanesulfonic acid (PFOS) disrupt pancreatic organogenesis in the zebrafish, Danio rerio
- PMID: 27810111
- PMCID: PMC5140685
- DOI: 10.1016/j.envpol.2016.10.057
Embryonic exposures to perfluorooctanesulfonic acid (PFOS) disrupt pancreatic organogenesis in the zebrafish, Danio rerio
Abstract
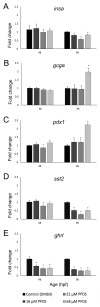
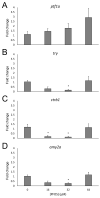
Perfluorooctanesulfonic acid (PFOS) is a ubiquitous environmental contaminant, previously utilized as a non-stick application for consumer products and firefighting foam. It can cross the placenta, and has been repeatedly associated with increased risk for diabetes in epidemiological studies. Here, we sought to establish the hazard posed by embryonic PFOS exposures on the developing pancreas in a model vertebrate embryo, and develop criteria for an adverse outcome pathway (AOP) framework to study the developmental origins of metabolic dysfunction. Zebrafish (Danio rerio) embryos were exposed to 16, 32, or 64 μM PFOS beginning at the mid-blastula transition. We assessed embryo health, size, and islet morphology in Tg(insulin-GFP) embryos at 48, 96 and 168 hpf, and pancreas length in Tg(ptf1a-GFP) embryos at 96 and 168 hpf. QPCR was used to measure gene expression of endocrine and exocrine hormones, digestive peptides, and transcription factors to determine whether these could be used as a predictive measure in an AOP. Embryos exposed to PFOS showed anomalous islet morphology and decreased islet size and pancreas length in a U-shaped dose-response curve, which resemble congenital defects associated with increased risk for diabetes in humans. Expression of genes encoding islet hormones and exocrine digestive peptides followed a similar pattern, as did total larval growth. Our results demonstrate that embryonic PFOS exposures can disrupt pancreatic organogenesis in ways that mimic human congenital defects known to predispose individuals to diabetes; however, future study of the association between these defects and metabolic dysfunction are needed to establish an improved AOP framework.
Keywords: Embryo; Exocrine pancreas; Insulin; Islets; Pancreas development; β cells.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures


References
-
- Agabi JO, Akhigbe AO. Comparative sonographic evaluation of the anteroposterior dimensions of the pancreas in diabetics and nondiabetics. Niger J Clin Pract. 2016;19:175–181. - PubMed
-
- Balakrishnan V, Narayanan VA, Siyad I, Radhakrishnan L, Nair P. Agenesis of the dorsal pancreas with chronic calcific pancreatitis. case report, review of the literature and genetic basis. JOP. 2006;7:651–659. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

