Probiotic With or Without Fiber Controls Body Fat Mass, Associated With Serum Zonulin, in Overweight and Obese Adults-Randomized Controlled Trial
- PMID: 27810310
- PMCID: PMC5264483
- DOI: 10.1016/j.ebiom.2016.10.036
Probiotic With or Without Fiber Controls Body Fat Mass, Associated With Serum Zonulin, in Overweight and Obese Adults-Randomized Controlled Trial
Abstract
Background: The gut microbiota is interlinked with obesity, but direct evidence of effects of its modulation on body fat mass is still scarce. We investigated the possible effects of Bifidobacterium animalisssp. lactis 420 (B420) and the dietary fiber Litesse® Ultra polydextrose (LU) on body fat mass and other obesity-related parameters.
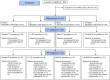
Methods: 225 healthy volunteers (healthy, BMI 28-34.9) were randomized into four groups (1:1:1:1), using a computer-generated sequence, for 6months of double-blind, parallel treatment: 1) Placebo, microcrystalline cellulose, 12g/d; 2) LU, 12g/d; 3) B420, 1010CFU/d in microcrystalline cellulose, 12g/d; 4) LU+B420, 12g+1010CFU/d. Body composition was monitored with dual-energy X-ray absorptiometry, and the primary outcome was relative change in body fat mass, comparing treatment groups to Placebo. Other outcomes included anthropometric measurements, food intake and blood and fecal biomarkers. The study was registered in Clinicaltrials.gov (NCT01978691).
Findings: There were marked differences in the results of the Intention-To-Treat (ITT; n=209) and Per Protocol (PP; n=134) study populations. The PP analysis included only those participants who completed the intervention with >80% product compliance and no antibiotic use. In addition, three participants were excluded from DXA analyses for PP due to a long delay between the end of intervention and the last DXA measurement. There were no significant differences between groups in body fat mass in the ITT population. However, LU+B420 and B420 seemed to improve weight management in the PP population. For relative change in body fat mass, LU+B420 showed a-4.5% (-1.4kg, P=0.02, N=37) difference to the Placebo group, whereas LU (+0.3%, P=1.00, N=35) and B420 (-3.0%, P=0.28, N=24) alone had no effect (overall ANOVA P=0.095, Placebo N=35). A post-hoc factorial analysis was significant for B420 (-4.0%, P=0.002 vs. Placebo). Changes in fat mass were most pronounced in the abdominal region, and were reflected by similar changes in waist circumference. B420 and LU+B420 also significantly reduced energy intake compared to Placebo. Changes in blood zonulin levels and hsCRP were associated with corresponding changes in trunk fat mass in the LU+B420 group and in the overall population. There were no differences between groups in the incidence of adverse events.
Discussion: This clinical trial demonstrates that a probiotic product with or without dietary fiber controls body fat mass. B420 and LU+B420 also reduced waist circumference and food intake, whereas LU alone had no effect on the measured outcomes.
Keywords: Clinical trial; Fiber; Obesity; Prebiotic; Probiotic; Synbiotic.
Copyright © 2016 The Authors. Published by Elsevier B.V. All rights reserved.
Figures


References
-
- Amar J., Chabo C., Waget A., Klopp P., Vachoux C., Bermudez-Humaran L.G., Smirnova N., Berge M., Sulpice T., Lahtinen S., Ouwehand A., Langella P., Rautonen N., Sansonetti P.J., Burcelin R. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol. Med. 2011;3:559–572. - PMC - PubMed
-
- Brillon D.J., Zheng B., Campbell R.G., Matthews D.E. Effect of cortisol on energy expenditure and amino acid metabolism in humans. Am. J. Phys. 1995;268:E501–E513. - PubMed
-
- Brun P., Castagliuolo I., Di Leo V., Buda A., Pinzani M., Palu G., Martines D. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G518–G525. - PubMed
-
- Burcelin R., Luche E., Serino M., Amar J. The gut microbiota ecology: a new opportunity for the treatment of metabolic diseases? Front. Biosci. (Landmark Ed.) 2009;14:5107–5117. - PubMed
-
- Cani P.D., Amar J., Iglesias M.A., Poggi M., Knauf C., Bastelica D., Neyrinck A.M., Fava F., Tuohy K.M., Chabo C., Waget A., Delmee E., Cousin B., Sulpice T., Chamontin B., Ferrieres J., Tanti J.F., Gibson G.R., Casteilla L., Delzenne N.M., Alessi M.C., Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

