Chemogenomics knowledgebase and systems pharmacology for hallucinogen target identification-Salvinorin A as a case study
- PMID: 27810775
- PMCID: PMC5327504
- DOI: 10.1016/j.jmgm.2016.08.001
Chemogenomics knowledgebase and systems pharmacology for hallucinogen target identification-Salvinorin A as a case study
Abstract
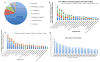
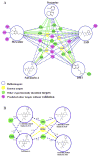
Drug abuse is a serious problem worldwide. Recently, hallucinogens have been reported as a potential preventative and auxiliary therapy for substance abuse. However, the use of hallucinogens as a drug abuse treatment has potential risks, as the fundamental mechanisms of hallucinogens are not clear. So far, no scientific database is available for the mechanism research of hallucinogens. We constructed a hallucinogen-specific chemogenomics database by collecting chemicals, protein targets and pathways closely related to hallucinogens. This information, together with our established computational chemogenomics tools, such as TargetHunter and HTDocking, provided a one-step solution for the mechanism study of hallucinogens. We chose salvinorin A, a potent hallucinogen extracted from the plant Salvia divinorum, as an example to demonstrate the usability of our platform. With the help of HTDocking program, we predicted four novel targets for salvinorin A, including muscarinic acetylcholine receptor 2, cannabinoid receptor 1, cannabinoid receptor 2 and dopamine receptor 2. We looked into the interactions between salvinorin A and the predicted targets. The binding modes, pose and docking scores indicate that salvinorin A may interact with some of these predicted targets. Overall, our database enriched the information of systems pharmacological analysis, target identification and drug discovery for hallucinogens.
Keywords: Chemogenomics database; Drug abuse; Hallucinogen; Salvinorin A; Systems pharmacology; Target identification.
Copyright © 2016. Published by Elsevier Inc.
Figures


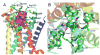
Similar articles
-
A unique natural selective kappa-opioid receptor agonist, salvinorin A, and its roles in human therapeutics.Phytochemistry. 2017 May;137:9-14. doi: 10.1016/j.phytochem.2017.02.001. Epub 2017 Feb 10. Phytochemistry. 2017. PMID: 28190678
-
Neuropharmacology of the naturally occurring kappa-opioid hallucinogen salvinorin A.Pharmacol Rev. 2011 Jun;63(2):316-47. doi: 10.1124/pr.110.003244. Epub 2011 Mar 28. Pharmacol Rev. 2011. PMID: 21444610 Free PMC article. Review.
-
Intramolecular transacetylation in salvinorins D and E.J Nat Prod. 2010 Apr 23;73(4):707-8. doi: 10.1021/np900447w. J Nat Prod. 2010. PMID: 20337449
-
Synthesis and κ-opioid receptor activity of furan-substituted salvinorin A analogues.J Med Chem. 2014 Dec 26;57(24):10464-75. doi: 10.1021/jm501521d. Epub 2014 Dec 9. J Med Chem. 2014. PMID: 25426797 Free PMC article.
-
Salvia divinorum and the unique diterpene hallucinogen, Salvinorin (divinorin) A.J Psychoactive Drugs. 1994 Jul-Sep;26(3):277-83. doi: 10.1080/02791072.1994.10472441. J Psychoactive Drugs. 1994. PMID: 7844657 Review.
Cited by
-
Reverse Screening Methods to Search for the Protein Targets of Chemopreventive Compounds.Front Chem. 2018 May 9;6:138. doi: 10.3389/fchem.2018.00138. eCollection 2018. Front Chem. 2018. PMID: 29868550 Free PMC article. Review.
-
Salvinorin A Does Not Affect Seizure Threshold in Mice.Molecules. 2020 Mar 7;25(5):1204. doi: 10.3390/molecules25051204. Molecules. 2020. PMID: 32155979 Free PMC article.
-
MCCS: a novel recognition pattern-based method for fast track discovery of anti-SARS-CoV-2 drugs.Brief Bioinform. 2021 Mar 22;22(2):946-962. doi: 10.1093/bib/bbaa260. Brief Bioinform. 2021. PMID: 33078827 Free PMC article.
-
Cutting-Edge Search for Safer Opioid Pain Relief: Retrospective Review of Salvinorin A and Its Analogs.Front Psychiatry. 2019 Mar 27;10:157. doi: 10.3389/fpsyt.2019.00157. eCollection 2019. Front Psychiatry. 2019. PMID: 30971961 Free PMC article. Review.
-
Computational Systems Pharmacology-Target Mapping for Fentanyl-Laced Cocaine Overdose.ACS Chem Neurosci. 2019 Aug 21;10(8):3486-3499. doi: 10.1021/acschemneuro.9b00109. Epub 2019 Jul 15. ACS Chem Neurosci. 2019. PMID: 31257858 Free PMC article.
References
-
- Center NDI. The Economic Impact of Illicit Drug Use on American Society. Washington DC: United States Department of Justice; 2011.
-
- Tan G, Lou Z, Jing J, Li W, Zhu Z, Zhao L, Zhang G, Chai Y. Screening and analysis of aconitum alkaloids and their metabolites in rat urine after oral administration of aconite roots extract using LC-TOFMS-based metabolomics. Biomedical chromatography : BMC. 2011;25(12):1343. - PubMed
-
- Nichols DE. Hallucinogens. Pharmacology & therapeutics. 2004;101(2):131–81. - PubMed
-
- Vargas-Perez H, Doblin R. The potential of psychedelics as a preventative and auxiliary therapy for drug abuse. Current drug abuse reviews. 2013;6(1):1–231. - PubMed
-
- Dyck E. Hitting Highs at Rock Bottom‘: LSD Treatment for Alcoholism, 1950–1970. Social History of Medicine. 2006;19(2):313–29.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

