Independent modes of ganglion cell translocation ensure correct lamination of the zebrafish retina
- PMID: 27810916
- PMCID: PMC5084647
- DOI: 10.1083/jcb.201604095
Independent modes of ganglion cell translocation ensure correct lamination of the zebrafish retina
Abstract
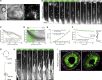
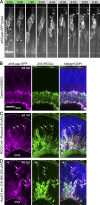
The arrangement of neurons into distinct layers is critical for neuronal connectivity and function. During development, most neurons move from their birthplace to the appropriate layer, where they polarize. However, kinetics and modes of many neuronal translocation events still await exploration. In this study, we investigate retinal ganglion cell (RGC) translocation across the embryonic zebrafish retina. After completing their translocation, RGCs establish the most basal retinal layer where they form the optic nerve. Using in toto light sheet microscopy, we show that somal translocation of RGCs is a fast and directed event. It depends on basal process attachment and stabilized microtubules. Interestingly, interference with somal translocation induces a switch to multipolar migration. This multipolar mode is less efficient but still leads to successful RGC layer formation. When both modes are inhibited though, RGCs fail to translocate and induce lamination defects. This indicates that correct RGC translocation is crucial for subsequent retinal lamination.
© 2016 Icha et al.
Figures






References
-
- Bellion A., Baudoin J.-P., Alvarez C., Bornens M., and Métin C.. 2005. Nucleokinesis in tangentially migrating neurons comprises two alternating phases: forward migration of the Golgi/centrosome associated with centrosome splitting and myosin contraction at the rear. J. Neurosci. 25:5691–5699. 10.1523/JNEUROSCI.1030-05.2005 - DOI - PMC - PubMed
-
- Brown N.L., Kanekar S., Vetter M.L., Tucker P.K., Gemza D.L., and Glaser T.. 1998. Math5 encodes a murine basic helix-loop-helix transcription factor expressed during early stages of retinal neurogenesis. Development. 125:4821–4833. - PubMed
-
- Cajal S.R. 1972. The Structure of the Retina. Thorpe S.A., and Glickstein M., editors. Charles Thomas, Springfield, Illinois. 196 pp.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

