Neurophysiological Organization of the Middle Face Patch in Macaque Inferior Temporal Cortex
- PMID: 27810930
- PMCID: PMC5157113
- DOI: 10.1523/JNEUROSCI.0237-16.2016
Neurophysiological Organization of the Middle Face Patch in Macaque Inferior Temporal Cortex
Abstract
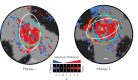
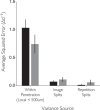
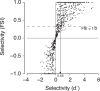
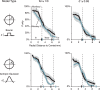
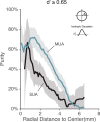
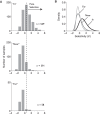
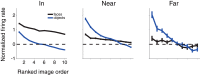
While early cortical visual areas contain fine scale spatial organization of neuronal properties, such as orientation preference, the spatial organization of higher-level visual areas is less well understood. The fMRI demonstration of face-preferring regions in human ventral cortex and monkey inferior temporal cortex ("face patches") raises the question of how neural selectivity for faces is organized. Here, we targeted hundreds of spatially registered neural recordings to the largest fMRI-identified face-preferring region in monkeys, the middle face patch (MFP), and show that the MFP contains a graded enrichment of face-preferring neurons. At its center, as much as 93% of the sites we sampled responded twice as strongly to faces than to nonface objects. We estimate the maximum neurophysiological size of the MFP to be ∼6 mm in diameter, consistent with its previously reported size under fMRI. Importantly, face selectivity in the MFP varied strongly even between neighboring sites. Additionally, extremely face-selective sites were ∼40 times more likely to be present inside the MFP than outside. These results provide the first direct quantification of the size and neural composition of the MFP by showing that the cortical tissue localized to the fMRI defined region consists of a very high fraction of face-preferring sites near its center, and a monotonic decrease in that fraction along any radial spatial axis.
Significance statement: The underlying organization of neurons that give rise to the large spatial regions of activity observed with fMRI is not well understood. Neurophysiological studies that have targeted the fMRI identified face patches in monkeys have provided evidence for both large-scale clustering and a heterogeneous spatial organization. Here we used a novel x-ray imaging system to spatially map the responses of hundreds of sites in and around the middle face patch. We observed that face-selective signal localized to the middle face patch was characterized by a gradual spatial enrichment. Furthermore, strongly face-selective sites were ∼40 times more likely to be found inside the patch than outside of the patch.
Keywords: faces; inferior temporal cortex; monkey; neurophysiology; spatial organization.
Copyright © 2016 the authors 0270-6474/16/3612729-17$15.00/0.
Figures







Similar articles
-
Probabilistic and single-subject retinotopic maps reveal the topographic organization of face patches in the macaque cortex.J Neurosci. 2014 Jul 30;34(31):10156-67. doi: 10.1523/JNEUROSCI.2914-13.2013. J Neurosci. 2014. PMID: 25080579 Free PMC article.
-
Large-scale, high-resolution neurophysiological maps underlying FMRI of macaque temporal lobe.J Neurosci. 2013 Sep 18;33(38):15207-19. doi: 10.1523/JNEUROSCI.1248-13.2013. J Neurosci. 2013. PMID: 24048850 Free PMC article.
-
The effect of face inversion for neurons inside and outside fMRI-defined face-selective cortical regions.J Neurophysiol. 2015 Mar 1;113(5):1644-55. doi: 10.1152/jn.00700.2014. Epub 2014 Dec 17. J Neurophysiol. 2015. PMID: 25520434 Free PMC article.
-
Neurophysiological mechanisms underlying face processing within and beyond the temporal cortical visual areas.Philos Trans R Soc Lond B Biol Sci. 1992 Jan 29;335(1273):11-20; discussion 20-1. doi: 10.1098/rstb.1992.0002. Philos Trans R Soc Lond B Biol Sci. 1992. PMID: 1348130 Review.
-
[Neural Mechanisms Underlying the Face Inversion Effect].Brain Nerve. 2015 Oct;67(10):1231-9. doi: 10.11477/mf.1416200288. Brain Nerve. 2015. PMID: 26450075 Review. Japanese.
Cited by
-
Parallel functional subnetworks embedded in the macaque face patch system.Sci Adv. 2022 Mar 11;8(10):eabm2054. doi: 10.1126/sciadv.abm2054. Epub 2022 Mar 9. Sci Adv. 2022. PMID: 35263138 Free PMC article.
-
Anatomical correlates of face patches in macaque inferotemporal cortex.Proc Natl Acad Sci U S A. 2020 Dec 22;117(51):32667-32678. doi: 10.1073/pnas.2018780117. Epub 2020 Dec 4. Proc Natl Acad Sci U S A. 2020. PMID: 33277435 Free PMC article.
-
Audiovisual integration in macaque face patch neurons.Curr Biol. 2021 May 10;31(9):1826-1835.e3. doi: 10.1016/j.cub.2021.01.102. Epub 2021 Feb 25. Curr Biol. 2021. PMID: 33636119 Free PMC article.
-
One object, two networks? Assessing the relationship between the face and body-selective regions in the primate visual system.Brain Struct Funct. 2022 May;227(4):1423-1438. doi: 10.1007/s00429-021-02420-7. Epub 2021 Nov 18. Brain Struct Funct. 2022. PMID: 34792643 Review.
-
Invariance of object detection in untrained deep neural networks.Front Comput Neurosci. 2022 Nov 3;16:1030707. doi: 10.3389/fncom.2022.1030707. eCollection 2022. Front Comput Neurosci. 2022. PMID: 36405785 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
