Small-molecule factor D inhibitors selectively block the alternative pathway of complement in paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome
- PMID: 27810992
- PMCID: PMC5394948
- DOI: 10.3324/haematol.2016.153312
Small-molecule factor D inhibitors selectively block the alternative pathway of complement in paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome
Abstract
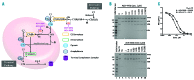
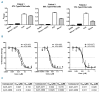
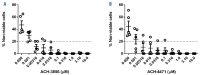
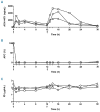
Paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome are diseases of excess activation of the alternative pathway of complement that are treated with eculizumab, a humanized monoclonal antibody against the terminal complement component C5. Eculizumab must be administered intravenously, and moreover some patients with paroxysmal nocturnal hemoglobinuria on eculizumab have symptomatic extravascular hemolysis, indicating an unmet need for additional therapeutic approaches. We report the activity of two novel small-molecule inhibitors of the alternative pathway component Factor D using in vitro correlates of both paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome. Both compounds bind human Factor D with high affinity and effectively inhibit its proteolytic activity against purified Factor B in complex with C3b. When tested using the traditional Ham test with cells from paroxysmal nocturnal hemoglobinuria patients, the Factor D inhibitors significantly reduced complement-mediated hemolysis at concentrations as low as 0.01 μM. Additionally the compound ACH-4471 significantly decreased C3 fragment deposition on paroxysmal nocturnal hemoglobinuria erythrocytes, indicating a reduced potential relative to eculizumab for extravascular hemolysis. Using the recently described modified Ham test with serum from patients with atypical hemolytic uremic syndrome, the compounds reduced the alternative pathway-mediated killing of PIGA-null reagent cells, thus establishing their potential utility for this disease of alternative pathway of complement dysregulation and validating the modified Ham test as a system for pre-clinical drug development for atypical hemolytic uremic syndrome. Finally, ACH-4471 blocked alternative pathway activity when administered orally to cynomolgus monkeys. In conclusion, the small-molecule Factor D inhibitors show potential as oral therapeutics for human diseases driven by the alternative pathway of complement, including paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome.
Copyright© Ferrata Storti Foundation.
Figures

References
-
- Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344(14):1058–1066. - PubMed
-
- Cataland SR, Wu HM. Diagnosis and management of complement mediated thrombotic microangiopathies. Blood Rev. 2014; 28(2):67–74. - PubMed
-
- Brodsky RA. Complement in hemolytic anemia. Blood. 2015;126(22):2459–2465. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

