Efficient acquisition of iron confers greater tolerance to saline-alkaline stress in rice (Oryza sativa L.)
- PMID: 27811002
- PMCID: PMC5181582
- DOI: 10.1093/jxb/erw407
Efficient acquisition of iron confers greater tolerance to saline-alkaline stress in rice (Oryza sativa L.)
Abstract
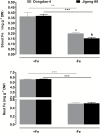
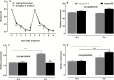
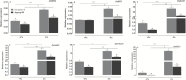
To elucidate the mechanisms underlying tolerance to saline-alkaline stress in two rice genotypes, Dongdao-4 and Jigeng-88, we exposed them to medium supplemented with 10 mM Na2CO3 and 40 mM NaCl (pH 8.5). Dongdao-4 plants displayed higher biomass, chlorophyll content, and photosynthetic rates, and a larger root system than Jigeng-88 under saline-alkaline conditions. Dongdao-4 had a higher shoot Na+/K+ ratio than Jigeng-88 under both control and saline-alkaline conditions. Dongdao-4 exhibited stronger rhizospheric acidification than Jigeng-88 under saline-alkaline conditions, resulting from greater up-regulation of H+-ATPases at the transcriptional level. Moreover, Fe concentrations in shoots and roots of Dongdao-4 were higher than those in Jigeng-88, and a higher rate of phytosiderophore exudation was detected in Dongdao-4 versus Jigeng-88 under saline-alkaline conditions. The Fe-deficiency-responsive genes OsIRO2, OsIRT1, OsNAS1, OsNAS2, OsYSL2, and OsYSL15 were more strongly up-regulated in Dongdao-4 than Jigeng-88 plants in saline-alkaline medium, implying greater tolerance of Dongdao-4 plants to Fe deficiency. To test this hypothesis, we compared the effects of Fe deficiency on the two genotypes, and found that Dongdao-4 was more tolerant to Fe deficiency. Exposure to Fe-deficient medium led to greater rhizospheric acidification and phytosiderophore exudation in Dongdao-4 than Jigeng-88 plants. Expression levels of OsIRO2, OsIRT1, OsNAS1, OsNAS2, OsYSL2, and OsYSL15 were higher in Dongdao-4 than Jigeng-88 plants under Fe-deficient conditions. These results demonstrate that a highly efficient Fe acquisition system together with a large root system may underpin the greater tolerance of Dongdao-4 plants to saline-alkaline stress.
Keywords: Oryza sativa; Dongdao-4; Jigeng-88; iron acquisition; iron deficiency; rice; saline-alkaline stress..
© The Author 2016. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures










References
-
- Abadia J, Vazquez S, Rellan-Alvarez R, et al. 2011. Towards a knowledge-based correction of iron chlorosis. Plant Physiology and Biochemistry 48, 471–482. - PubMed
-
- Apse MP, Aharon GS, Snedden WA, Blumwald E. 1999. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in arabidopsis. Science 285, 1256–1258. - PubMed
-
- Awad F, Römheld V, Marschner H. 1988. Mobilization of ferric iron from a calcareous soil by plant-borne chelators (Phytosiderophores). Journal of Plant Nutrition 11, 701–713.
-
- Babgohari MZ, Niazi A, Moghadam AA, Deihimi T, Ebrahimie E. 2013. Genome-wide analysis of key salinity-tolerance transporter (HKT1;5) in wheat and wild wheat relatives (A and D genomes). In Vitro Cellular & Developmental Biology – Plant 49, 97–106.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

