A randomised controlled trial of tiotropium in adolescents with severe symptomatic asthma
- PMID: 27811070
- PMCID: PMC5298196
- DOI: 10.1183/13993003.01100-2016
A randomised controlled trial of tiotropium in adolescents with severe symptomatic asthma
Abstract
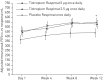
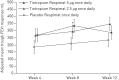
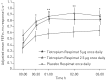
We present results from the first phase III trial of once-daily tiotropium add-on to inhaled corticosteroids (ICS) plus one or more controller therapies in adolescents with severe symptomatic asthma.In this double-blind, parallel-group trial (NCT01277523), 392 patients aged 12-17 years were randomised to receive once-daily tiotropium 5 µg or 2.5 µg, or placebo, as an add-on to ICS plus other controller therapies over 12 weeks. The primary and key secondary end-points were change from baseline (response) in peak forced expiratory volume in 1 s (FEV1) within 3 h post-dosing (FEV1(0-3h)) and trough FEV1, respectively, after 12 weeks of treatment.Tiotropium 5 µg provided numerical improvements in peak FEV1(0-3h) response, compared with placebo (90 mL; p=0.104), and significant improvements were observed with tiotropium 2.5 µg (111 mL; p=0.046). Numerical improvements in trough FEV1 response and asthma control were observed with both tiotropium doses, compared with placebo. The safety and tolerability of tiotropium were comparable with those of placebo.Once-daily tiotropium Respimat add-on to ICS plus one or more controller therapies in adolescents with severe symptomatic asthma was well tolerated. The primary end-point of efficacy was not met, although positive trends for improvements in lung function and asthma control were observed.
Copyright ©ERS 2017.
Conflict of interest statement
can be found alongside this article at erj.ersjournals.com
Figures


Comment in
-
Tiotropium in paediatric asthma.Eur Respir J. 2017 Jan 11;49(1):1602034. doi: 10.1183/13993003.02034-2016. Print 2017 Jan. Eur Respir J. 2017. PMID: 28077480 No abstract available.
References
-
- Asthma UK. Asthma Facts and FAQs. www.asthma.org.uk/asthma-facts-and-statistics. Date last updated: 2014. Date last accessed: May 19, 2016.
-
- Schmier JK, Manjunath R, Halpern MT, et al. . The impact of inadequately controlled asthma in urban children on quality of life and productivity. Ann Allergy Asthma Immunol 2007; 98: 245–251. - PubMed
-
- Bateman ED, Boushey HA, Bousquet J, et al. . Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med 2004; 170: 836–844. - PubMed
-
- Demoly P, Paggiaro P, Plaza V, et al. . Prevalence of asthma control among adults in France, Germany, Italy, Spain and the UK. Eur Respir Rev 2009; 18: 105–112. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
