Injury-induced ctgfa directs glial bridging and spinal cord regeneration in zebrafish
- PMID: 27811277
- PMCID: PMC5114142
- DOI: 10.1126/science.aaf2679
Injury-induced ctgfa directs glial bridging and spinal cord regeneration in zebrafish
Abstract
Unlike mammals, zebrafish efficiently regenerate functional nervous system tissue after major spinal cord injury. Whereas glial scarring presents a roadblock for mammalian spinal cord repair, glial cells in zebrafish form a bridge across severed spinal cord tissue and facilitate regeneration. We performed a genome-wide profiling screen for secreted factors that are up-regulated during zebrafish spinal cord regeneration. We found that connective tissue growth factor a (ctgfa) is induced in and around glial cells that participate in initial bridging events. Mutations in ctgfa disrupted spinal cord repair, and transgenic ctgfa overexpression or local delivery of human CTGF recombinant protein accelerated bridging and functional regeneration. Our study reveals that CTGF is necessary and sufficient to stimulate glial bridging and natural spinal cord regeneration.
Copyright © 2016, American Association for the Advancement of Science.
Figures

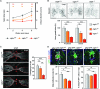
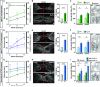
Comment in
-
Building bridges to regenerate axons.Science. 2016 Nov 4;354(6312):544-545. doi: 10.1126/science.aal2112. Science. 2016. PMID: 27811250 No abstract available.
References
-
- Becker T, Wullimann MF, Becker CG, Bernhardt RR, Schachner M. Axonal regrowth after spinal cord transection in adult zebrafish. J Comp Neurol. 1997;377:577–595. - PubMed
-
- Hui SP, Dutta A, Ghosh S. Cellular response after crush injury in adult zebrafish spinal cord. Dev Dyn. 2010;239:2962–2979. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

