Long-term exposure to Myozyme results in a decrease of anti-drug antibodies in late-onset Pompe disease patients
- PMID: 27812025
- PMCID: PMC5096052
- DOI: 10.1038/srep36182
Long-term exposure to Myozyme results in a decrease of anti-drug antibodies in late-onset Pompe disease patients
Abstract
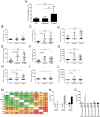
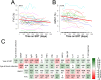
Immunogenicity of recombinant human acid-alpha glucosidase (rhGAA) in enzyme replacement therapy (ERT) is a safety and efficacy concern in the management of late-onset Pompe disease (LOPD). However, long-term effects of ERT on humoral and cellular responses to rhGAA are still poorly understood. To better understand the impact of immunogenicity of rhGAA on the efficacy of ERT, clinical data and blood samples from LOPD patients undergoing ERT for >4 years (n = 28) or untreated (n = 10) were collected and analyzed. In treated LOPD patients, anti-rhGAA antibodies peaked within the first 1000 days of ERT, while long-term exposure to rhGAA resulted in clearance of antibodies with residual production of non-neutralizing IgG. Analysis of T cell responses to rhGAA showed detectable T cell reactivity only after in vitro restimulation. Upregulation of several cytokines and chemokines was detectable in both treated and untreated LOPD subjects, while IL2 secretion was detectable only in subjects who received ERT. These results indicate that long-term ERT in LOPD patients results in a decrease in antibody titers and residual production of non-inhibitory IgGs. Immune responses to GAA following long-term ERT do not seem to affect efficacy of ERT and are consistent with an immunomodulatory effect possibly mediated by regulatory T cells.
Conflict of interest statement
Pascal Laforet has received grants and honoraria from Genzyme Company, and honoraria from BioMarin and Amicus Therapeutics and all the other authors declare no competing financial interests.
Figures




References
-
- Hirschhorn R. a. R. AJJ. In The Metabolic and Molecular Basis of Inherited Disease (ed Beaudet A. L., Scriver C. R., Sly W. S., Valle D.) 3389–3420 (McGraw-Hill, 2001).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

