A Foxp2 Mutation Implicated in Human Speech Deficits Alters Sequencing of Ultrasonic Vocalizations in Adult Male Mice
- PMID: 27812326
- PMCID: PMC5071336
- DOI: 10.3389/fnbeh.2016.00197
A Foxp2 Mutation Implicated in Human Speech Deficits Alters Sequencing of Ultrasonic Vocalizations in Adult Male Mice
Abstract
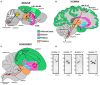
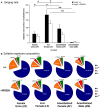
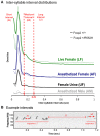
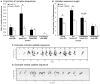
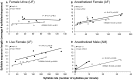
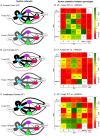
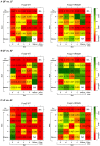
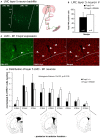
Development of proficient spoken language skills is disrupted by mutations of the FOXP2 transcription factor. A heterozygous missense mutation in the KE family causes speech apraxia, involving difficulty producing words with complex learned sequences of syllables. Manipulations in songbirds have helped to elucidate the role of this gene in vocal learning, but findings in non-human mammals have been limited or inconclusive. Here, we performed a systematic study of ultrasonic vocalizations (USVs) of adult male mice carrying the KE family mutation. Using novel statistical tools, we found that Foxp2 heterozygous mice did not have detectable changes in USV syllable acoustic structure, but produced shorter sequences and did not shift to more complex syntax in social contexts where wildtype animals did. Heterozygous mice also displayed a shift in the position of their rudimentary laryngeal motor cortex (LMC) layer-5 neurons. Our findings indicate that although mouse USVs are mostly innate, the underlying contributions of FoxP2 to sequencing of vocalizations are conserved with humans.
Keywords: FoxP2; KE family; song; speech apraxia; syntax; ultrasonic vocalizations.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

