Overexpression of Mcl-1 exacerbates lymphocyte accumulation and autoimmune kidney disease in lpr mice
- PMID: 27813531
- PMCID: PMC5344201
- DOI: 10.1038/cdd.2016.125
Overexpression of Mcl-1 exacerbates lymphocyte accumulation and autoimmune kidney disease in lpr mice
Abstract
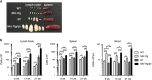
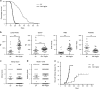
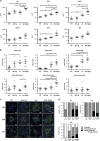
Cell death by apoptosis has a critical role during embryonic development and in maintaining tissue homeostasis. In mammals, there are two converging apoptosis pathways: the 'extrinsic' pathway, which is triggered by engagement of cell surface 'death receptors' such as Fas/APO-1; and the 'intrinsic' pathway, which is triggered by diverse cellular stresses, and is regulated by pro-survival and pro-apoptotic members of the Bcl-2 family of proteins. Pro-survival Mcl-1, which can block activation of the pro-apoptotic proteins, Bax and Bak, appears critical for the survival and maintenance of multiple haemopoietic cell types. To investigate the impact on haemopoiesis of simultaneously inhibiting both apoptosis pathways, we introduced the vavP-Mcl-1 transgene, which causes overexpression of Mcl-1 protein in all haemopoietic lineages, into Faslpr/lpr mice, which lack functional Fas and are prone to autoimmunity. The combined mutations had a modest impact on myelopoiesis, primarily an increase in the macrophage/monocyte population in Mcl-1tg/lpr mice compared with lpr or Mcl-1tg mice. The impact on lymphopoiesis was striking, with a marked elevation in all major lymphoid subsets, including the non-conventional double-negative (DN) T cells (TCRβ+CD4-CD8-B220+) characteristic of Faslpr/lpr mice. Of note, the onset of autoimmunity was markedly accelerated in Mcl-1tg/lpr mice compared with lpr mice, and this was preceded by an increase in immunoglobulin (Ig)-producing cells and circulating autoantibodies. This degree of impact was surprising, given the relatively mild phenotype conferred by the vavP-Mcl-1 transgene by itself: a two- to threefold elevation of peripheral B and T cells, no significant increase in the non-conventional DN T-cell population and no autoimmune disease. Comparison of the phenotype with that of other susceptible mice suggests that the development of autoimmune disease in Mcl-1tg/lpr mice may be influenced not only by Ig-producing cells but also other haemopoietic cell types.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol 2014; 15: 49–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

