Enterovirus D-68 Infection, Prophylaxis, and Vaccination in a Novel Permissive Animal Model, the Cotton Rat (Sigmodon hispidus)
- PMID: 27814404
- PMCID: PMC5096705
- DOI: 10.1371/journal.pone.0166336
Enterovirus D-68 Infection, Prophylaxis, and Vaccination in a Novel Permissive Animal Model, the Cotton Rat (Sigmodon hispidus)
Abstract
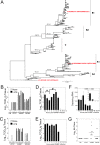
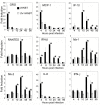
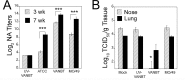
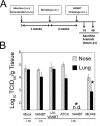
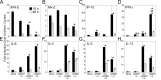
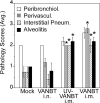
In recent years, there has been a significant increase in detection of Enterovirus D-68 (EV-D68) among patients with severe respiratory infections worldwide. EV-D68 is now recognized as a re-emerging pathogen; however, due to lack of a permissive animal model for EV-D68, a comprehensive understanding of the pathogenesis and immune response against EV-D68 has been hampered. Recently, it was shown that EV-D68 has a strong affinity for α2,6-linked sialic acids (SAs) and we have shown previously that α2,6-linked SAs are abundantly present in the respiratory tract of cotton rats (Sigmodon hispidus). Thus, we hypothesized that cotton rats could be a potential model for EV-D68 infection. Here, we evaluated the ability of two recently isolated EV-D68 strains (VANBT/1 and MO/14/49), along with the historical prototype Fermon strain (ATCC), to infect cotton rats. We found that cotton rats are permissive to EV-D68 infection without virus adaptation. The different strains of EV-D68 showed variable infection profiles and the ability to produce neutralizing antibody (NA) upon intranasal infection or intramuscular immunization. Infection with the VANBT/1 resulted in significant induction of pulmonary cytokine gene expression and lung pathology. Intramuscular immunization with live VANBT/1 or MO/14/49 induced strong homologous antibody responses, but a moderate heterologous NA response. We showed that passive prophylactic administration of serum with high content of NA against VANBT/1 resulted in an efficient antiviral therapy. VANBT/1-immunized animals showed complete protection from VANBT/1 challenge, but induced strong pulmonary Th1 and Th2 cytokine responses and enhanced lung pathology, indicating the generation of exacerbated immune response by immunization. In conclusion, our data illustrate that the cotton rat is a powerful animal model that provides an experimental platform to investigate pathogenesis, immune response, anti-viral therapies and vaccines against EV-D68 infection.
Conflict of interest statement
JCGB and MSB are co-founders and serves as President and Chief Scientific Officer for Sigmovir Biosystems, Inc., respectively. All other authors declare no conflict of interest. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

Similar articles
-
Enterovirus D68 infection in cotton rats results in systemic inflammation with detectable viremia associated with extracellular vesicle and neurologic disease.Sci Rep. 2025 Feb 22;15(1):6514. doi: 10.1038/s41598-025-89447-6. Sci Rep. 2025. PMID: 39987168 Free PMC article.
-
Single B cells reveal the antibody responses of rhesus macaques immunized with an inactivated enterovirus D68 vaccine.Arch Virol. 2020 Aug;165(8):1777-1789. doi: 10.1007/s00705-020-04676-6. Epub 2020 May 28. Arch Virol. 2020. PMID: 32462286 Free PMC article.
-
Nasal Infection of Enterovirus D68 Leading to Lower Respiratory Tract Pathogenesis in Ferrets (Mustela putorius furo).Viruses. 2017 May 10;9(5):104. doi: 10.3390/v9050104. Viruses. 2017. PMID: 28489053 Free PMC article.
-
Current Understanding of Humoral Immunity to Enterovirus D68.J Pediatric Infect Dis Soc. 2018 Dec 26;7(suppl_2):S49-S53. doi: 10.1093/jpids/piy124. J Pediatric Infect Dis Soc. 2018. PMID: 30590621 Review.
-
Global reemergence of enterovirus D68 as an important pathogen for acute respiratory infections.Rev Med Virol. 2015 Mar;25(2):102-14. doi: 10.1002/rmv.1820. Epub 2014 Dec 3. Rev Med Virol. 2015. PMID: 25471236 Free PMC article. Review.
Cited by
-
SARS-CoV-2 infection augments species- and age-specific predispositions in cotton rats.Sci Rep. 2023 Jan 14;13(1):757. doi: 10.1038/s41598-022-27328-y. Sci Rep. 2023. PMID: 36641520 Free PMC article.
-
Enterovirus D68 infection induces IL-17-dependent neutrophilic airway inflammation and hyperresponsiveness.JCI Insight. 2018 Aug 23;3(16):e121882. doi: 10.1172/jci.insight.121882. eCollection 2018 Aug 23. JCI Insight. 2018. PMID: 30135310 Free PMC article.
-
Respiratory infection with Enterovirus D68 induces severe acute lung inflammation in the pediatric ferret model.iScience. 2025 Jul 5;28(8):113071. doi: 10.1016/j.isci.2025.113071. eCollection 2025 Aug 15. iScience. 2025. PMID: 40727933 Free PMC article.
-
Advances in the Treatment of Enterovirus-D68 and Rhinovirus Respiratory Infections.Infect Dis Rep. 2025 Jun 1;17(3):61. doi: 10.3390/idr17030061. Infect Dis Rep. 2025. PMID: 40559192 Free PMC article. Review.
-
Small Animal Models of Respiratory Viral Infection Related to Asthma.Viruses. 2018 Dec 1;10(12):682. doi: 10.3390/v10120682. Viruses. 2018. PMID: 30513770 Free PMC article. Review.
References
-
- Kono R, Sasagawa A, Ishii K, Sugiura S, Ochi M. Pandemic of new type of conjunctivitis. Lancet. 1972;1(7762):1191–4. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

