Myofibroblasts and lung fibrosis induced by carbon nanotube exposure
- PMID: 27814727
- PMCID: PMC5097370
- DOI: 10.1186/s12989-016-0172-2
Myofibroblasts and lung fibrosis induced by carbon nanotube exposure
Abstract
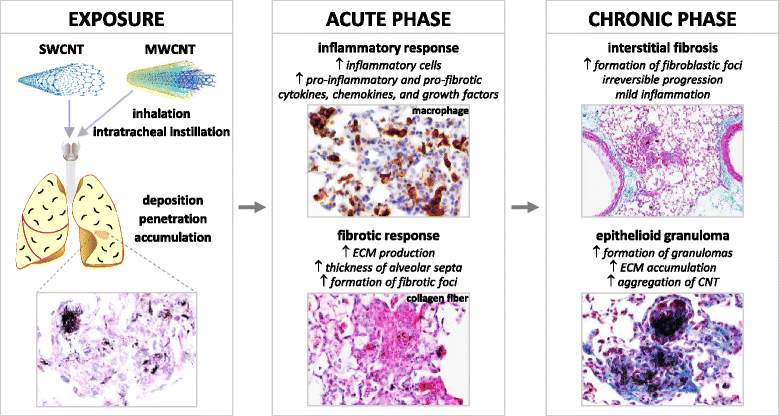
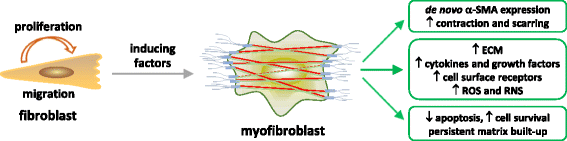
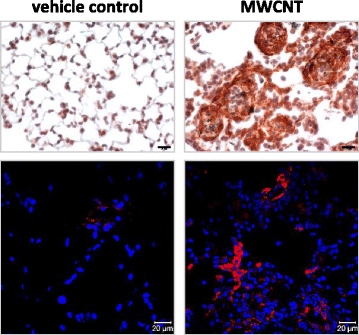
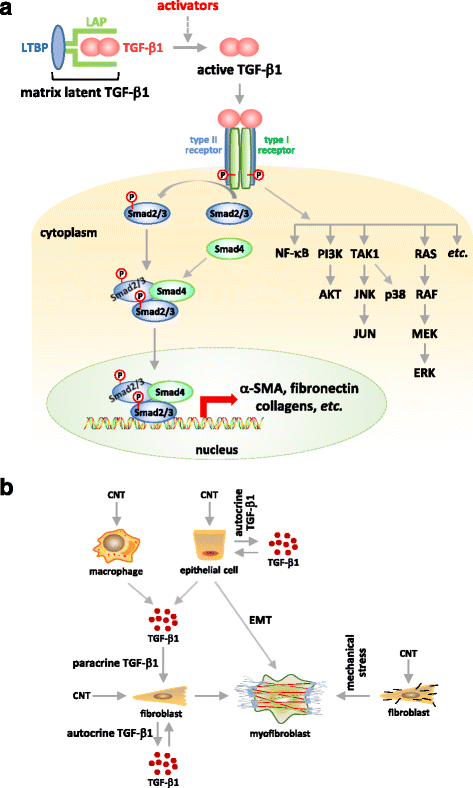
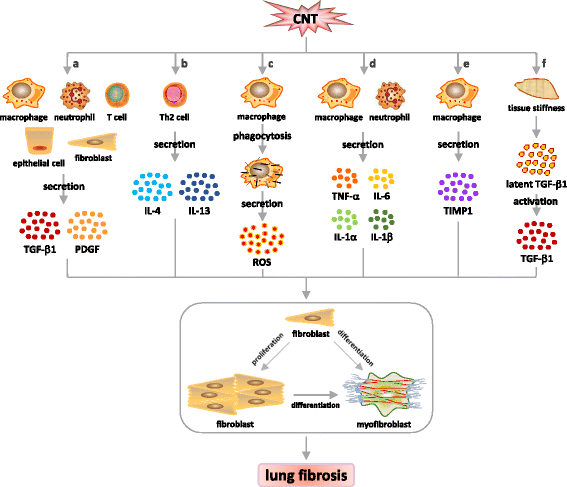
Carbon nanotubes (CNTs) are newly developed materials with unique properties and a range of industrial and commercial applications. A rapid expansion in the production of CNT materials may increase the risk of human exposure to CNTs. Studies in rodents have shown that certain forms of CNTs are potent fibrogenic inducers in the lungs to cause interstitial, bronchial, and pleural fibrosis characterized by the excessive deposition of collagen fibers and the scarring of involved tissues. The cellular and molecular basis underlying the fibrotic response to CNT exposure remains poorly understood. Myofibroblasts are a major type of effector cells in organ fibrosis that secrete copious amounts of extracellular matrix proteins and signaling molecules to drive fibrosis. Myofibroblasts also mediate the mechano-regulation of fibrotic matrix remodeling via contraction of their stress fibers. Recent studies reveal that exposure to CNTs induces the differentiation of myofibroblasts from fibroblasts in vitro and stimulates pulmonary accumulation and activation of myofibroblasts in vivo. Moreover, mechanistic analyses provide insights into the molecular underpinnings of myofibroblast differentiation and function induced by CNTs in the lungs.In view of the apparent fibrogenic activity of CNTs and the emerging role of myofibroblasts in the development of organ fibrosis, we discuss recent findings on CNT-induced lung fibrosis with emphasis on the role of myofibroblasts in the pathologic development of lung fibrosis. Particular attention is given to the formation and activation of myofibroblasts upon CNT exposure and the possible mechanisms by which CNTs regulate the function and dynamics of myofibroblasts in the lungs. It is evident that a fundamental understanding of the myofibroblast and its function and regulation in lung fibrosis will have a major influence on the future research on the pulmonary response to nano exposure, particle and fiber-induced pneumoconiosis, and other human lung fibrosing diseases.
Keywords: Animal model; Carbon nanotube; Extracellular matrix; Lung fibrosis; Mechanism; Myofibroblast.
Figures
Similar articles
-
Osteopontin enhances multi-walled carbon nanotube-triggered lung fibrosis by promoting TGF-β1 activation and myofibroblast differentiation.Part Fibre Toxicol. 2017 Jun 8;14(1):18. doi: 10.1186/s12989-017-0198-0. Part Fibre Toxicol. 2017. PMID: 28595626 Free PMC article.
-
Carbon nanotubes and crystalline silica induce matrix remodeling and contraction by stimulating myofibroblast transformation in a three-dimensional culture of human pulmonary fibroblasts: role of dimension and rigidity.Arch Toxicol. 2018 Nov;92(11):3291-3305. doi: 10.1007/s00204-018-2306-9. Epub 2018 Sep 18. Arch Toxicol. 2018. PMID: 30229330
-
Multiwalled carbon nanotubes induce a fibrogenic response by stimulating reactive oxygen species production, activating NF-κB signaling, and promoting fibroblast-to-myofibroblast transformation.Chem Res Toxicol. 2011 Dec 19;24(12):2237-48. doi: 10.1021/tx200351d. Epub 2011 Nov 22. Chem Res Toxicol. 2011. PMID: 22081859
-
Type 2 Immune Mechanisms in Carbon Nanotube-Induced Lung Fibrosis.Front Immunol. 2018 May 22;9:1120. doi: 10.3389/fimmu.2018.01120. eCollection 2018. Front Immunol. 2018. PMID: 29872441 Free PMC article. Review.
-
Signaling Pathways Implicated in Carbon Nanotube-Induced Lung Inflammation.Front Immunol. 2020 Dec 11;11:552613. doi: 10.3389/fimmu.2020.552613. eCollection 2020. Front Immunol. 2020. PMID: 33391253 Free PMC article. Review.
Cited by
-
[Effect of low-concentration paclitaxel on collagen deposition outside rat pulmonary artery smooth muscle cells and related mechanism].Zhongguo Dang Dai Er Ke Za Zhi. 2019 Sep;21(9):924-929. doi: 10.7499/j.issn.1008-8830.2019.09.016. Zhongguo Dang Dai Er Ke Za Zhi. 2019. PMID: 31506155 Free PMC article. Chinese.
-
Osteopontin enhances multi-walled carbon nanotube-triggered lung fibrosis by promoting TGF-β1 activation and myofibroblast differentiation.Part Fibre Toxicol. 2017 Jun 8;14(1):18. doi: 10.1186/s12989-017-0198-0. Part Fibre Toxicol. 2017. PMID: 28595626 Free PMC article.
-
TRPM7 restrains plasmin activity and promotes transforming growth factor-β1 signaling in primary human lung fibroblasts.Arch Toxicol. 2022 Oct;96(10):2767-2783. doi: 10.1007/s00204-022-03342-x. Epub 2022 Jul 21. Arch Toxicol. 2022. PMID: 35864199 Free PMC article.
-
Role of A2B adenosine receptor-dependent adenosine signaling in multi-walled carbon nanotube-triggered lung fibrosis in mice.J Nanobiotechnology. 2019 Mar 29;17(1):45. doi: 10.1186/s12951-019-0478-y. J Nanobiotechnology. 2019. PMID: 30922349 Free PMC article.
-
Macrophage polarization and activation at the interface of multi-walled carbon nanotube-induced pulmonary inflammation and fibrosis.Nanotoxicology. 2018 Mar;12(2):153-168. doi: 10.1080/17435390.2018.1425501. Epub 2018 Jan 16. Nanotoxicology. 2018. PMID: 29338488 Free PMC article.
References
-
- Husain AN, Kumar V. The lung. In: Kumar V, Abbas AK, Fausto N, editors. Robbins and Cotran Pathologic Basis of Disease. 7. Philadelphia: Elsevier Saunders; 2005. pp. 711–772.
-
- NIOSH . Health Effects of Occupational Exposure to Respirable Crystalline Silica. DHHS (NIOSH) Publication No. 2002-129. DHHS CDC NIOSH: Cincinnati; 2002.
-
- Morgan WKC, Seaton A. Occupational lung diseases. 3. Philadelphia: W.B. Saunders Company; 1995.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

