Prions, Chaperones, and Proteostasis in Yeast
- PMID: 27815300
- PMCID: PMC5287078
- DOI: 10.1101/cshperspect.a023663
Prions, Chaperones, and Proteostasis in Yeast
Abstract
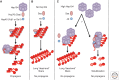
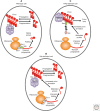
Prions are alternatively folded, self-perpetuating protein isoforms involved in a variety of biological and pathological processes. Yeast prions are protein-based heritable elements that serve as an excellent experimental system for studying prion biology. The propagation of yeast prions is controlled by the same Hsp104/70/40 chaperone machinery that is involved in the protection of yeast cells against proteotoxic stress. Ribosome-associated chaperones, proteolytic pathways, cellular quality-control compartments, and cytoskeletal networks influence prion formation, maintenance, and toxicity. Environmental stresses lead to asymmetric prion distribution in cell divisions. Chaperones and cytoskeletal proteins mediate this effect. Overall, this is an intimate relationship with the protein quality-control machinery of the cell, which enables prions to be maintained and reproduced. The presence of many of these same mechanisms in higher eukaryotes has implications for the diagnosis and treatment of mammalian amyloid diseases.
Copyright © 2017 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


References
-
- Aguilaniu H, Gustafsson L, Rigoulet M, Nyström T. 2003. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science 299: 1751–1753. - PubMed
-
- Allen KD, Chernova TA, Tennant EP, Wilkinson KD, Chernoff YO. 2007. Effects of ubiquitin system alterations on the formation and loss of a yeast prion. J Biol Chem 282: 3004–3013. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
