Altered hippocampal arteriole structure and function in a rat model of preeclampsia: Potential role in impaired seizure-induced hyperemia
- PMID: 27815419
- PMCID: PMC5536792
- DOI: 10.1177/0271678X16676287
Altered hippocampal arteriole structure and function in a rat model of preeclampsia: Potential role in impaired seizure-induced hyperemia
Abstract
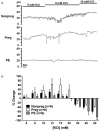
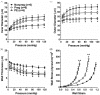
We investigated the effect of experimental preeclampsia on hyperemia during seizure in the hippocampus and vascular function and structure of hippocampal arterioles using Sprague Dawley rats (n = 14/group) that were nonpregnant, pregnant (d20), or had experimental preeclampsia (induced by a high cholesterol diet d7-20). Hyperemia was measured via hydrogen clearance basally and during pentylenetetrazol-induced seizure (40-130 mg/kg i.v.). Reactivity of isolated and pressurized hippocampal arterioles to KCl, nitric oxide synthase inhibition with NG-nitro-L-arginine methyl ester and the nitric oxide donor sodium nitroprusside were investigated. Capillary density was quantified via immunohistochemistry. Cerebral blood flow increased during seizure vs. baseline in pregnant (118 ± 14 vs. 87 ± 9 mL/100 g/min; p < 0.05) and nonpregnant rats (106 ± 9 vs. 82 ± 9 mL/100 g/min; p < 0.05) but was unchanged in preeclamptic rats (79 ± 16 vs. 91 ± 4 mL/100 g/min; p > 0.05), suggesting impaired seizure-induced hyperemia in preeclampsia. Hippocampal arterioles from preeclamptic rats had less basal tone, and dilated less to 15 mM KCl (9 ± 8%) vs. pregnant (61 ± 27%) and nonpregnant rats (20 ± 11%). L-NAME had no effect on hippocampal arterioles in any group, but dilation to sodium nitroprusside was similar. Structurally, hippocampal arterioles from preeclamptic rats underwent inward hypotrophic remodeling and capillary rarefaction. Impaired seizure-induced hyperemia, vascular dysfunction, and limited vasodilatory reserve of hippocampal arterioles could potentiate hippocampal injury in preeclampsia especially during eclampsia.
Keywords: Cerebral blood flow; cerebral vascular function; hippocampus; preeclampsia/eclampsia; seizure.
Figures





References
-
- Lindheimer MD, Taylor RN, Roberts JM, et al. Introduction, history, controversies, and definitions. In: Taylor RN, Roberts JM, Cunningham FG, et al. (eds). Chesley's hypertensive disorders in pregnancy, 4th ed Boston: Academic Press/Elsevier, 2014.
-
- Roberts JM, Redman CW. Pre-eclampsia: more than pregnancy-induced hypertension. Lancet 1993; 341: 1447–1451. - PubMed
-
- MacKay AP, Berg CJ, Atrash HK. Pregnancy-related mortality from preeclampsia and eclampsia. Obstet Gynecol 2001; 97: 533–538. - PubMed
-
- Zeeman G, Cipolla M, Cunningham G. Cerebrovascular (patho) physiology in preeclampsia/eclampsia. In: Lindhiemer M, Roberts J, Cunningham G. (eds). Chesley's hypertensive disorders in pregnancy, San Diego, CA: Elsevier, 2009, pp. 227–248.
-
- Shah AK, Rajamani K, Whitty JE. Eclampsia: a neurological perspective. J Neurol Sci 2008; 271: 158–167. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

