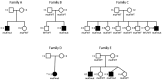
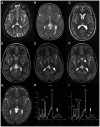
MECR Mutations Cause Childhood-Onset Dystonia and Optic Atrophy, a Mitochondrial Fatty Acid Synthesis Disorder
- PMID: 27817865
- PMCID: PMC5142118
- DOI: 10.1016/j.ajhg.2016.09.021
MECR Mutations Cause Childhood-Onset Dystonia and Optic Atrophy, a Mitochondrial Fatty Acid Synthesis Disorder
Abstract
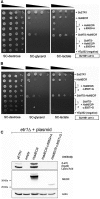
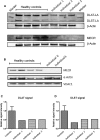
Mitochondrial fatty acid synthesis (mtFAS) is an evolutionarily conserved pathway essential for the function of the respiratory chain and several mitochondrial enzyme complexes. We report here a unique neurometabolic human disorder caused by defective mtFAS. Seven individuals from five unrelated families presented with childhood-onset dystonia, optic atrophy, and basal ganglia signal abnormalities on MRI. All affected individuals were found to harbor recessive mutations in MECR encoding the mitochondrial trans-2-enoyl-coenzyme A-reductase involved in human mtFAS. All six mutations are extremely rare in the general population, segregate with the disease in the families, and are predicted to be deleterious. The nonsense c.855T>G (p.Tyr285∗), c.247_250del (p.Asn83Hisfs∗4), and splice site c.830+2_830+3insT mutations lead to C-terminal truncation variants of MECR. The missense c.695G>A (p.Gly232Glu), c.854A>G (p.Tyr285Cys), and c.772C>T (p.Arg258Trp) mutations involve conserved amino acid residues, are located within the cofactor binding domain, and are predicted by structural analysis to have a destabilizing effect. Yeast modeling and complementation studies validated the pathogenicity of the MECR mutations. Fibroblast cell lines from affected individuals displayed reduced levels of both MECR and lipoylated proteins as well as defective respiration. These results suggest that mutations in MECR cause a distinct human disorder of the mtFAS pathway. The observation of decreased lipoylation raises the possibility of a potential therapeutic strategy.
Copyright © 2016 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Schneider S.A., Bhatia K.P. Secondary dystonia--clinical clues and syndromic associations. Eur. J. Neurol. 2010;17(Suppl 1):52–57. - PubMed
-
- Zuccoli G., Yannes M.P., Nardone R., Bailey A., Goldstein A. Bilateral symmetrical basal ganglia and thalamic lesions in children: an update (2015) Neuroradiology. 2015;57:973–989. - PubMed
-
- Meyer E., Kurian M.A., Hayflick S.J. Neurodegeneration with brain iron accumulation: genetic diversity and pathophysiological mechanisms. Annu. Rev. Genomics Hum. Genet. 2015;16:257–279. - PubMed
-
- Lake N.J., Compton A.G., Rahman S., Thorburn D.R. Leigh syndrome: One disorder, more than 75 monogenic causes. Ann. Neurol. 2016;79:190–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

