A surrogate marker of piperaquine-resistant Plasmodium falciparum malaria: a phenotype-genotype association study
- PMID: 27818097
- PMCID: PMC5266792
- DOI: 10.1016/S1473-3099(16)30415-7
A surrogate marker of piperaquine-resistant Plasmodium falciparum malaria: a phenotype-genotype association study
Erratum in
-
Corrections.Lancet Infect Dis. 2018 Aug;18(8):829. doi: 10.1016/S1473-3099(18)30396-7. Epub 2018 Jun 12. Lancet Infect Dis. 2018. PMID: 29907497 Free PMC article. No abstract available.
Abstract
Background: Western Cambodia is the epicentre of Plasmodium falciparum multidrug resistance and is facing high rates of dihydroartemisinin-piperaquine treatment failures. Genetic tools to detect the multidrug-resistant parasites are needed. Artemisinin resistance can be tracked using the K13 molecular marker, but no marker exists for piperaquine resistance. We aimed to identify genetic markers of piperaquine resistance and study their association with dihydroartemisinin-piperaquine treatment failures.
Methods: We obtained blood samples from Cambodian patients infected with P falciparum and treated with dihydroartemisinin-piperaquine. Patients were followed up for 42 days during the years 2009-15. We established in-vitro and ex-vivo susceptibility profiles for a subset using piperaquine survival assays. We determined whole-genome sequences by Illumina paired-reads sequencing, copy number variations by qPCR, RNA concentrations by qRT-PCR, and protein concentrations by immunoblotting. Fisher's exact and non-parametric Wilcoxon rank-sum tests were used to identify significant differences in single-nucleotide polymorphisms or copy number variants, respectively, for differential distribution between piperaquine-resistant and piperaquine-sensitive parasite lines.
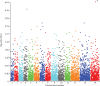
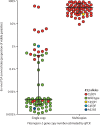
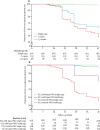
Findings: Whole-genome exon sequence analysis of 31 culture-adapted parasite lines associated amplification of the plasmepsin 2-plasmepsin 3 gene cluster with in-vitro piperaquine resistance. Ex-vivo piperaquine survival assay profiles of 134 isolates correlated with plasmepsin 2 gene copy number. In 725 patients treated with dihydroartemisinin-piperaquine, multicopy plasmepsin 2 in the sample collected before treatment was associated with an adjusted hazard ratio (aHR) for treatment failure of 20·4 (95% CI 9·1-45·5, p<0·0001). Multicopy plasmepsin 2 predicted dihydroartemisinin-piperaquine failures with 0·94 (95% CI 0·88-0·98) sensitivity and 0·77 (0·74-0·81) specificity. Analysis of samples collected across the country from 2002 to 2015 showed that the geographical and temporal increase of the proportion of multicopy plasmepsin 2 parasites was highly correlated with increasing dihydroartemisinin-piperaquine treatment failure rates (r=0·89 [95% CI 0·77-0·95], p<0·0001, Spearman's coefficient of rank correlation). Dihydroartemisinin-piperaquine efficacy at day 42 fell below 90% when the proportion of multicopy plasmepsin 2 parasites exceeded 22%.
Interpretation: Piperaquine resistance in Cambodia is strongly associated with amplification of plasmepsin 2-3, encoding haemoglobin-digesting proteases, regardless of the location. Multicopy plasmepsin 2 constitutes a surrogate molecular marker to track piperaquine resistance. A molecular toolkit combining plasmepsin 2 with K13 and mdr1 monitoring should provide timely information for antimalarial treatment and containment policies.
Funding: Institut Pasteur in Cambodia, Institut Pasteur Paris, National Institutes of Health, WHO, Agence Nationale de la Recherche, Investissement d'Avenir programme, Laboratoire d'Excellence Integrative "Biology of Emerging Infectious Diseases".
Copyright This is an Open Access article published under the CC BY 3.0 IGO license which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In any use of this article, there should be no suggestion that WHO endorses any specific organisation, products or services. The use of the WHO logo is not permitted. This notice should be preserved along with the article’s original URL.
Figures

Comment in
-
New genetic marker for piperaquine resistance in Plasmodium falciparum.Lancet Infect Dis. 2017 Feb;17(2):119-121. doi: 10.1016/S1473-3099(16)30414-5. Epub 2016 Nov 3. Lancet Infect Dis. 2017. PMID: 27818096 No abstract available.
References
-
- Noedl H, Se Y, Schaecher K, et al. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–2620. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

