Auxin Acts through MONOPTEROS to Regulate Plant Cell Polarity and Pattern Phyllotaxis
- PMID: 27818174
- PMCID: PMC5154752
- DOI: 10.1016/j.cub.2016.09.044
Auxin Acts through MONOPTEROS to Regulate Plant Cell Polarity and Pattern Phyllotaxis
Abstract
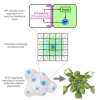
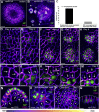
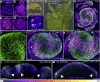
The periodic formation of plant organs such as leaves and flowers gives rise to intricate patterns that have fascinated biologists and mathematicians alike for hundreds of years [1]. The plant hormone auxin plays a central role in establishing these patterns by promoting organ formation at sites where it accumulates due to its polar, cell-to-cell transport [2-6]. Although experimental evidence as well as modeling suggest that feedback from auxin to its transport direction may help specify phyllotactic patterns [7-12], the nature of this feedback remains unclear [13]. Here we reveal that polarization of the auxin efflux carrier PIN-FORMED 1 (PIN1) is regulated by the auxin response transcription factor MONOPTEROS (MP) [14]. We find that in the shoot, cell polarity patterns follow MP expression, which in turn follows auxin distribution patterns. By perturbing MP activity both globally and locally, we show that localized MP activity is necessary for the generation of polarity convergence patterns and that localized MP expression is sufficient to instruct PIN1 polarity directions non-cell autonomously, toward MP-expressing cells. By expressing MP in the epidermis of mp mutants, we further show that although MP activity in a single-cell layer is sufficient to promote polarity convergence patterns, MP in sub-epidermal tissues helps anchor these polarity patterns to the underlying cells. Overall, our findings reveal a patterning module in plants that determines organ position by orienting transport of the hormone auxin toward cells with high levels of MP-mediated auxin signaling. We propose that this feedback process acts broadly to generate periodic plant architectures.
Keywords: ARF5; Arabidopsis; MONOPTEROS; PIN1; auxin; cell polarity; organ positioning; organogenesis; pattern formation; phyllotaxis.
Copyright © 2016 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


Comment in
-
Phyllotaxis: A Matthew Effect in Auxin Action.Curr Biol. 2016 Dec 5;26(23):R1233-R1235. doi: 10.1016/j.cub.2016.10.019. Curr Biol. 2016. PMID: 27923132
References
-
- Jean R.V., Barabé D. World Scientific; 1998. Symmetry in Plants.
-
- Reinhardt D., Pesce E.R., Stieger P., Mandel T., Baltensperger K., Bennett M., Traas J., Friml J., Kuhlemeier C. Regulation of phyllotaxis by polar auxin transport. Nature. 2003;426:255–260. - PubMed
-
- Benková E., Michniewicz M., Sauer M., Teichmann T., Seifertová D., Jürgens G., Friml J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

