Pharmacogenetics and Predictive Testing of Drug Hypersensitivity Reactions
- PMID: 27818635
- PMCID: PMC5073094
- DOI: 10.3389/fphar.2016.00396
Pharmacogenetics and Predictive Testing of Drug Hypersensitivity Reactions
Abstract
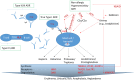
Adverse drug reactions adverse drug reaction (ADR) occur in approximately 17% of patients. Avoiding ADR is thus mandatory from both an ethical and an economic point of view. Whereas, pharmacogenetics changes of the pharmacokinetics may contribute to the explanation of some type A reactions, strong relationships of genetic markers has also been shown for drug hypersensitivity belonging to type B reactions. We present the classifications of ADR, discuss genetic influences and focus on delayed-onset hypersensitivity reactions, i.e., drug-induced liver injury, drug-induced agranulocytosis, and severe cutaneous ADR. A guidance how to read and interpret the contingency table is provided as well as an algorithm whether and how a test for a pharmacogenetic biomarker should be conducted.
Keywords: adverse drug reactions (ADRs); drug hypersensitivity reactions; drug-induced agranulocytosis (DIA); drug-induced liver injury (DILI); drug-induced severe cutaneous adverse reactions (SCARs).
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

