Origin of unusual bandgap shift and dual emission in organic-inorganic lead halide perovskites
- PMID: 27819049
- PMCID: PMC5091363
- DOI: 10.1126/sciadv.1601156
Origin of unusual bandgap shift and dual emission in organic-inorganic lead halide perovskites
Abstract
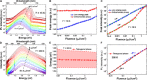
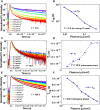
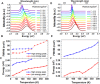
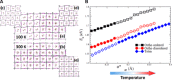
Emission characteristics of metal halide perovskites play a key role in the current widespread investigations into their potential uses in optoelectronics and photonics. However, a fundamental understanding of the molecular origin of the unusual blueshift of the bandgap and dual emission in perovskites is still lacking. In this direction, we investigated the extraordinary photoluminescence behavior of three representatives of this important class of photonic materials, that is, CH3NH3PbI3, CH3NH3PbBr3, and CH(NH2)2PbBr3, which emerged from our thorough studies of the effects of temperature on their bandgap and emission decay dynamics using time-integrated and time-resolved photoluminescence spectroscopy. The low-temperature (<100 K) photoluminescence of CH3NH3PbI3 and CH3NH3PbBr3 reveals two distinct emission peaks, whereas that of CH(NH2)2PbBr3 shows a single emission peak. Furthermore, irrespective of perovskite composition, the bandgap exhibits an unusual blueshift by raising the temperature from 15 to 300 K. Density functional theory and classical molecular dynamics simulations allow for assigning the additional photoluminescence peak to the presence of molecularly disordered orthorhombic domains and also rationalize that the unusual blueshift of the bandgap with increasing temperature is due to the stabilization of the valence band maximum. Our findings provide new insights into the salient emission properties of perovskite materials, which define their performance in solar cells and light-emitting devices.
Keywords: Hybrid perovskites; density functional theory; luminescence; photoluminescence; photovoltaics.
Figures

References
-
- Green M. A., Ho-Baillie A., Snaith H. J., The emergence of perovskite solar cells. Nat. Photonics 8, 506–514 (2014).
-
- Rau U., Reciprocity relation between photovoltaic quantum efficiency and electroluminescent emission of solar cells. Phys. Rev. B 76, 085303 (2007).
-
- Kojima A., Teshima K., Shirai Y., Miyasaka T., Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 131, 6050–6051 (2009). - PubMed
-
- Tan Z.-K., Moghaddam R. S., Lai M. L., Docampo P., Higler R., Deschler F., Price M., Sadhanala A., Pazos L. M., Credgington D., Hanusch F., Bein T., Snaith H. J., Friend R. H., Bright light-emitting diodes based on organometal halide perovskite. Nat. Nanotechnol. 9, 687–692 (2014). - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

