The Hidden Conundrum of Phosphoinositide Signaling in Cancer
- PMID: 27819060
- PMCID: PMC5094282
- DOI: 10.1016/j.trecan.2016.05.009
The Hidden Conundrum of Phosphoinositide Signaling in Cancer
Abstract
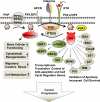
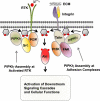
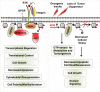
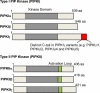
Phosphoinositide 3-kinase (PI3K) generation of PI(3,4,5)P3 from PI(4,5)P2 and the subsequent activation of Akt and its downstream signaling cascades (e.g. mTORC1) dominates the landscape of phosphoinositide signaling axis in cancer research. However, PI(4,5)P2 is breaking its boundary as merely a substrate for PI3K and phospholipase C (PLC), and is now an established lipid messenger pivotal for different cellular events in cancer. Here, we review the phosphoinositide signaling axis in cancer, giving due weight to PI(4,5)P2 and its generating enzymes, the phosphatidylinositol phosphate (PIP) kinases (PIPKs). We highlighted how PI(4,5)P2 and PIP kinases serve as a proximal node in phosphoinositide signaling axis and how its interaction with cytoskeletal proteins regulates migratory and invasive nexus of metastasizing tumor cells.
Keywords: Akt; PI(3,4,5)P3; PI(4,5)P2; PI3K; PIPKIγ.
Figures

References
-
- Kolch W, Halasz M, Granovskaya M, Kholodenko BN. The dynamic control of signal transduction networks in cancer cells. Nat Rev Cancer. 2015;15:515–527. - PubMed
-
- Vanhaesebroeck B, Stephens L, Hawkins P. PI3K signalling: the path to discovery and understanding. Nat Rev Mol Cell Biol. 2012;13:195–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
