LMP1 signaling pathway activates IRF4 in latent EBV infection and a positive circuit between PI3K and Src is required
- PMID: 27819673
- PMCID: PMC5685659
- DOI: 10.1038/onc.2016.380
LMP1 signaling pathway activates IRF4 in latent EBV infection and a positive circuit between PI3K and Src is required
Abstract
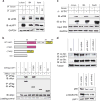
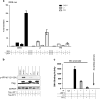
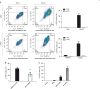
Interferon (IFN) regulatory factors (IRFs) have crucial roles in immune regulation and oncogenesis. We have recently shown that IRF4 is activated through c-Src-mediated tyrosine phosphorylation in virus-transformed cells. However, the intracellular signaling pathway triggering Src activation of IRF4 remains unknown. In this study, we provide evidence that Epstein-Barr virus (EBV) latent membrane protein 1 (LMP1) promotes IRF4 phosphorylation and markedly stimulates IRF4 transcriptional activity, and that Src mediates LMP1 activation of IRF4. As to more precise mechanism, we show that LMP1 physically interacts with c-Src, and the phosphatidylinositol 3 kinase (PI3K) subunit P85 mediates their interaction. Depletion of P85 by P85-specific short hairpin RNAs disrupts their interaction and diminishes IRF4 phosphorylation in EBV-transformed cells. Furthermore, we show that Src is upstream of PI3K for activation of both IRF4 and Akt. In turn, inhibition of PI3K kinase activity by the PI3K-speicfic inhibitor LY294002 impairs Src activity. Our results show that LMP1 signaling is responsible for IRF4 activation, and further characterize the IRF4 regulatory network that is a promising therapeutic target for specific hematological malignancies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Interferon regulatory factor 4 is activated through c-Src-mediated tyrosine phosphorylation in virus-transformed cells.J Virol. 2013 Sep;87(17):9672-9. doi: 10.1128/JVI.01435-13. Epub 2013 Jun 26. J Virol. 2013. PMID: 23804646 Free PMC article.
-
Src kinase and Syk activation initiate PI3K signaling by a chimeric latent membrane protein 1 in Epstein-Barr virus (EBV)+ B cell lymphomas.PLoS One. 2012;7(8):e42610. doi: 10.1371/journal.pone.0042610. Epub 2012 Aug 3. PLoS One. 2012. PMID: 22880054 Free PMC article.
-
EB-virus latent membrane protein 1 potentiates the stemness of nasopharyngeal carcinoma via preferential activation of PI3K/AKT pathway by a positive feedback loop.Oncogene. 2016 Jun 30;35(26):3419-31. doi: 10.1038/onc.2015.402. Epub 2015 Nov 16. Oncogene. 2016. PMID: 26568302
-
The Latent Membrane Protein 1 (LMP1).Curr Top Microbiol Immunol. 2015;391:119-49. doi: 10.1007/978-3-319-22834-1_4. Curr Top Microbiol Immunol. 2015. PMID: 26428373 Review.
-
Lineage- and Stage-Specific Oncogenicity of IRF4.Exp Hematol. 2022 Oct;114:9-17. doi: 10.1016/j.exphem.2022.07.300. Epub 2022 Jul 29. Exp Hematol. 2022. PMID: 35908629 Review.
Cited by
-
The master antioxidant defense is activated during EBV latent infection.J Virol. 2023 Nov 30;97(11):e0095323. doi: 10.1128/jvi.00953-23. Epub 2023 Oct 25. J Virol. 2023. PMID: 37877721 Free PMC article.
-
Environmental pollutants and phosphoinositide signaling in autoimmunity.J Hazard Mater. 2024 Mar 5;465:133080. doi: 10.1016/j.jhazmat.2023.133080. Epub 2023 Dec 1. J Hazard Mater. 2024. PMID: 38091799 Free PMC article. Review.
-
The multiple roles of interferon regulatory factor family in health and disease.Signal Transduct Target Ther. 2024 Oct 9;9(1):282. doi: 10.1038/s41392-024-01980-4. Signal Transduct Target Ther. 2024. PMID: 39384770 Free PMC article. Review.
-
Dasatinib exacerbates splenomegaly of mice inoculated with Epstein-Barr virus-infected lymphoblastoid cell lines.Sci Rep. 2020 Mar 9;10(1):4355. doi: 10.1038/s41598-020-61300-y. Sci Rep. 2020. PMID: 32152351 Free PMC article.
-
LIMD1 is induced by and required for LMP1 signaling, and protects EBV-transformed cells from DNA damage-induced cell death.Oncotarget. 2017 Dec 26;9(5):6282-6297. doi: 10.18632/oncotarget.23676. eCollection 2018 Jan 19. Oncotarget. 2017. PMID: 29464072 Free PMC article.
References
-
- Boshoff C, Weiss R. Aids-related malignancies. Nat Rev Cancer. 2002;2:373–382. - PubMed
-
- Carbone A, Gloghini A, Dotti G. EBV-associated lymphoproliferative disorders: classification and treatment. Oncologist. 2008;13:577–585. - PubMed
-
- Pagano JS, Blaser M, Buendia MA, Damania B, Khalili K, Raab-Traub N, et al. Infectious agents and cancer: criteria for a causal relation. Semin Cancer Biol. 2004;14:453–471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

