Molecular-level analysis of the serum antibody repertoire in young adults before and after seasonal influenza vaccination
- PMID: 27820605
- PMCID: PMC5301914
- DOI: 10.1038/nm.4224
Molecular-level analysis of the serum antibody repertoire in young adults before and after seasonal influenza vaccination
Abstract
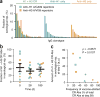
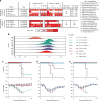
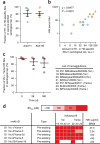
Molecular understanding of serological immunity to influenza has been confounded by the complexity of the polyclonal antibody response in humans. Here we used high-resolution proteomics analysis of immunoglobulin (referred to as Ig-seq) coupled with high-throughput sequencing of transcripts encoding B cell receptors (BCR-seq) to quantitatively determine the antibody repertoire at the individual clonotype level in the sera of young adults before and after vaccination with trivalent seasonal influenza vaccine. The serum repertoire comprised between 40 and 147 clonotypes that were specific to each of the three monovalent components of the trivalent influenza vaccine, with boosted pre-existing clonotypes accounting for ∼60% of the response. An unexpectedly high fraction of serum antibodies recognized both the H1 and H3 monovalent vaccines. Recombinant versions of these H1 + H3 cross-reactive antibodies showed broad binding to hemagglutinins (HAs) from previously circulating virus strains; several of these antibodies, which were prevalent in the serum of multiple donors, recognized the same conserved epitope in the HA head domain. Although the HA-head-specific H1 + H3 antibodies did not show neutralization activity in vitro, they protected mice against infection with the H1N1 and H3N2 virus strains when administered before or after challenge. Collectively, our data reveal unanticipated insights regarding the serological response to influenza vaccination and raise questions about the added benefits of using a quadrivalent vaccine instead of a trivalent vaccine.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Comment in
-
Understanding immune responses to the influenza vaccine.Nat Med. 2016 Dec 6;22(12):1387-1388. doi: 10.1038/nm.4248. Nat Med. 2016. PMID: 27923018 No abstract available.
References
-
- Molinari NAM, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25:5086–5096. - PubMed
-
- Lambert LC, Fauci AS. Influenza vaccines for the future. N Engl J Med. 2010;363:2036–2044. - PubMed
-
- Rimmelzwaan GF, McElhaney JE. Correlates of protection: novel generations of influenza vaccines. Vaccine. 2008;26:D41–D44. - PubMed
-
- Krammer F, Palese P. Advances in the development of influenza virus vaccines. Nat Rev Drug Discov. 2015;14:167–182. - PubMed
-
- Monto AS, et al. Comparative efficacy of inactivated and live attenuated influenza vaccines. N Engl J Med. 2009;361:1260–1267. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

