Single administration of Selective Internal Radiation Therapy versus continuous treatment with sorafeNIB in locally advanced hepatocellular carcinoma (SIRveNIB): study protocol for a phase iii randomized controlled trial
- PMID: 27821083
- PMCID: PMC5100089
- DOI: 10.1186/s12885-016-2868-y
Single administration of Selective Internal Radiation Therapy versus continuous treatment with sorafeNIB in locally advanced hepatocellular carcinoma (SIRveNIB): study protocol for a phase iii randomized controlled trial
Abstract
Background: Approximately 20 % of hepatocellular carcinoma (HCC) patients diagnosed in the early stages may benefit from potentially curative ablative therapies such as surgical resection, transplantation or radiofrequency ablation. For patients not eligible for such options, prognosis is poor. Sorafenib and Selective Internal Radiation Therapy (SIRT) are clinically proven treatment options in patients with unresectable HCC, and this study aims to assess overall survival following either SIRT or Sorafenib therapy for locally advanced HCC patients.
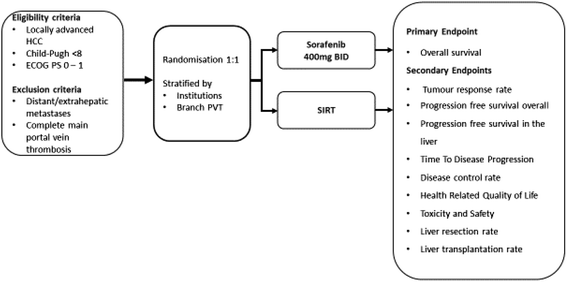
Methods: This investigator-initiated, multi-centre, open-label, randomized, controlled trial will enrol 360 patients with locally advanced HCC, as defined by Barcelona Clinic Liver Cancer stage B or stage C, without distant metastases, and which is not amenable to immediate curative treatment. Exclusion criteria include previous systemic therapy, metastatic disease, complete occlusion of the main portal vein, or a Child-Pugh score of >7. Eligible patients will be randomised 1:1 and stratified by centre and presence or absence of portal vein thrombosis to receive either a single administration of SIRT using yttrium-90 resin microspheres (SIR-Spheres®, Sirtex Medical Limited, Sydney, Australia) targeted at HCC in the liver by the trans-arterial route or continuous oral Sorafenib (Nexavar®, Bayer Pharma AG, Berlin, Germany) at a dose of 400 mg twice daily until disease progression, no further response, complete regression or unacceptable toxicity. Patients for both the Sorafenib and SIRT arms will be followed-up every 4 weeks for the first 3 months and 12 weekly thereafter. Overall survival is the primary endpoint, assessed for the intention-to-treat population. Secondary endpoints are tumour response rate, time-to-tumour progression, progression free survival, quality of life and down-staging to receive potentially curative therapy.
Discussion: Definitive data comparing these two therapies will help to determine clinical practice in the large group of patients with locally advanced HCC and improve outcomes for such patients.
Trial registration: ClinicalTrials.gov identifier, NCT01135056 , first received 24, May 2010.
Keywords: Advanced hepatocellular carcinoma; Asia-Pacific; Liver cancer; Phase III; Radioembolisation; Randomized controlled trial; SIR-Spheres; Selective internal radiation therapy; Sorafenib; Systemic therapy.
Figures
Similar articles
-
Radioembolisation with yttrium‒90 microspheres versus sorafenib for treatment of advanced hepatocellular carcinoma (SARAH): study protocol for a randomised controlled trial.Trials. 2014 Dec 3;15:474. doi: 10.1186/1745-6215-15-474. Trials. 2014. PMID: 25472660 Free PMC article. Clinical Trial.
-
Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial.Lancet Oncol. 2017 Dec;18(12):1624-1636. doi: 10.1016/S1470-2045(17)30683-6. Epub 2017 Oct 26. Lancet Oncol. 2017. PMID: 29107679 Clinical Trial.
-
Selective internal radiation therapies for unresectable early-, intermediate- or advanced-stage hepatocellular carcinoma: systematic review, network meta-analysis and economic evaluation.Health Technol Assess. 2020 Sep;24(48):1-264. doi: 10.3310/hta24480. Health Technol Assess. 2020. PMID: 33001024 Free PMC article.
-
Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma.J Hepatol. 2019 Dec;71(6):1164-1174. doi: 10.1016/j.jhep.2019.08.006. Epub 2019 Aug 14. J Hepatol. 2019. PMID: 31421157 Clinical Trial.
-
Treatment options for unresectable HCC with a focus on SIRT with Yttrium-90 resin microspheres.Int J Clin Pract. 2017 Nov;71(11). doi: 10.1111/ijcp.12972. Epub 2017 Jul 30. Int J Clin Pract. 2017. PMID: 28758319 Review.
Cited by
-
Stereotactic Body Radiotherapy vs Sorafenib Alone in Hepatocellular Carcinoma: The NRG Oncology/RTOG 1112 Phase 3 Randomized Clinical Trial.JAMA Oncol. 2025 Feb 1;11(2):136-144. doi: 10.1001/jamaoncol.2024.5403. JAMA Oncol. 2025. PMID: 39699905 Clinical Trial.
-
The different effects of adefovir dipivoxil and telbivudine on the prognosis of hepatitis b virus-related hepatocellular carcinoma patients after curative resection.Medicine (Baltimore). 2019 Feb;98(6):e14386. doi: 10.1097/MD.0000000000014386. Medicine (Baltimore). 2019. PMID: 30732177 Free PMC article.
-
VESPRO: An Individual Patient Data Prospective Meta-Analysis of Selective Internal Radiation Therapy Versus Sorafenib for Advanced, Locally Advanced, or Recurrent Hepatocellular Carcinoma of the SARAH and SIRveNIB Trials.JMIR Res Protoc. 2017 Feb 15;6(2):e17. doi: 10.2196/resprot.7016. JMIR Res Protoc. 2017. PMID: 28202430 Free PMC article.
-
Identifying optimal therapies in patients with advanced hepatocellular carcinoma: a systematic review and network meta-analysis.Transl Gastroenterol Hepatol. 2022 Oct 25;7:38. doi: 10.21037/tgh-20-318. eCollection 2022. Transl Gastroenterol Hepatol. 2022. PMID: 36300147 Free PMC article.
-
[Stereotactic body radiation therapy is superior to sorafenib in terms of survival in patients with hepatocellular carcinoma].Strahlenther Onkol. 2019 Feb;195(2):188-189. doi: 10.1007/s00066-018-1408-x. Strahlenther Onkol. 2019. PMID: 30552450 German. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical