Pharmacokinetics, Distribution, Metabolism, and Excretion of Omadacycline following a Single Intravenous or Oral Dose of 14C-Omadacycline in Rats
- PMID: 27821446
- PMCID: PMC5192155
- DOI: 10.1128/AAC.01784-16
Pharmacokinetics, Distribution, Metabolism, and Excretion of Omadacycline following a Single Intravenous or Oral Dose of 14C-Omadacycline in Rats
Abstract
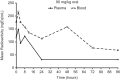
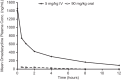
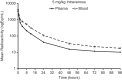
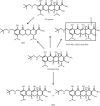
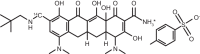
The absorption, distribution, metabolism, and excretion (ADME) of omadacycline, a first-in-class aminomethylcycline antibiotic with a broad spectrum of activity against Gram-positive, Gram-negative, anaerobic, and atypical bacteria, were evaluated in rats. Tissue distribution was investigated by quantitative whole-body autoradiography in male Long-Evans Hooded (LEH) rats. Following an intravenous (i.v.) dose of 5 mg/kg of body weight, radioactivity widely and rapidly distributed into most tissues. The highest tissue-to-blood concentration ratios (t/b) were observed in bone mineral, thyroid gland, and Harderian gland at 24 h post-i.v. dose. There was no evidence of stable accumulation in uveal tract tissue, suggesting the absence of a stable binding interaction with melanin. Following a 90 mg/kg oral dose in LEH rats, the highest t/b were observed in bone mineral, Harderian gland, liver, spleen, and salivary gland. The plasma protein binding levels were 26% in the rat and 15% to 21% in other species. Omadacycline plasma clearance was 1.2 liters/h/kg, and its half-life was 4.6 h; the steady-state volume of distribution (Vss) was 6.89 liters/kg. Major circulating components in plasma were intact omadacycline and its epimer. Consistent with observations in human, approximately 80% of the dose was excreted into the feces as unchanged omadacycline after i.v. administration. Fecal excretion was primarily the result of biliary excretion (∼40%) and direct gastrointestinal secretion (∼30%). However, urinary excretion (∼30%) was equally prominent after i.v. dosing.
Keywords: ADME; aminomethylcycline; animal models; omadacycline.
Copyright © 2016 Lin et al.
Figures
Similar articles
-
Randomized, Open-Label Study of the Pharmacokinetics and Safety of Oral and Intravenous Administration of Omadacycline to Healthy Subjects.Antimicrob Agents Chemother. 2016 Nov 21;60(12):7431-7435. doi: 10.1128/AAC.01393-16. Print 2016 Dec. Antimicrob Agents Chemother. 2016. PMID: 27736760 Free PMC article. Clinical Trial.
-
Safety and Pharmacokinetics of the Aminomethylcycline Antibiotic Omadacycline Administered to Healthy Subjects in Oral Multiple-Dose Regimens.Antimicrob Agents Chemother. 2018 Jan 25;62(2):e01487-17. doi: 10.1128/AAC.01487-17. Print 2018 Feb. Antimicrob Agents Chemother. 2018. PMID: 29180524 Free PMC article. Clinical Trial.
-
Clinical disposition, metabolism and in vitro drug-drug interaction properties of omadacycline.Xenobiotica. 2017 Aug;47(8):682-696. doi: 10.1080/00498254.2016.1213465. Epub 2016 Aug 8. Xenobiotica. 2017. PMID: 27499331
-
Omadacycline: A Novel Oral and Intravenous Aminomethylcycline Antibiotic Agent.Drugs. 2020 Feb;80(3):285-313. doi: 10.1007/s40265-020-01257-4. Drugs. 2020. PMID: 31970713 Review.
-
Absorption, distribution, metabolism and excretion of glucosamine sulfate. A review.Arzneimittelforschung. 2001 Sep;51(9):699-725. doi: 10.1055/s-0031-1300105. Arzneimittelforschung. 2001. PMID: 11642003 Review.
Cited by
-
Omadacycline efficacy in the hollow fibre system model of pulmonary Mycobacterium avium complex and potency at clinically attainable doses.J Antimicrob Chemother. 2022 May 29;77(6):1694-1705. doi: 10.1093/jac/dkac068. J Antimicrob Chemother. 2022. PMID: 35257162 Free PMC article.
-
Present and Future Perspectives on Therapeutic Options for Carbapenemase-Producing Enterobacterales Infections.Microorganisms. 2021 Mar 31;9(4):730. doi: 10.3390/microorganisms9040730. Microorganisms. 2021. PMID: 33807464 Free PMC article. Review.
-
Efficacy and safety of omadacycline for treating complicated skin and soft tissue infections: a meta-analysis of randomized controlled trials.BMC Infect Dis. 2024 Feb 19;24(1):219. doi: 10.1186/s12879-024-09097-3. BMC Infect Dis. 2024. PMID: 38374030 Free PMC article.
-
Pharmacokinetics and Pharmacodynamics of Oral and Intravenous Omadacycline.Clin Infect Dis. 2019 Aug 1;69(Suppl 1):S16-S22. doi: 10.1093/cid/ciz309. Clin Infect Dis. 2019. PMID: 31367744 Free PMC article. Review.
-
Activity of Omadacycline in Rat Methicillin-Resistant Staphylococcus aureus Osteomyelitis.Antimicrob Agents Chemother. 2022 Jan 18;66(1):e0170321. doi: 10.1128/AAC.01703-21. Epub 2021 Nov 1. Antimicrob Agents Chemother. 2022. PMID: 34723626 Free PMC article.
References
-
- Noel GJ, Draper MP, Hait H, Tanaka SK, Arbeit RD. 2012. A randomized, evaluator-blind, phase 2 study comparing the safety and efficacy of omadacycline to those of linezolid for treatment of complicated skin and skin structure infections. Antimicrob Agents Chemother 56:5650–5654. doi:10.1128/AAC.00948-12. - DOI - PMC - PubMed
-
- Sun H, Ting L, Maietta R, Machineni S, Praestgaard J, Kuemmell A, Stein DS, Sunkara G, Kovacs SJ, Villano S, Tanaka SK. October 2016. A randomized, open-label study of the pharmacokinetics and safety of oral and intravenous administration of omadacycline in healthy subjects. Antimicrob Agents Chemother doi:10.1128/AAC.01393-16. - DOI - PMC - PubMed
-
- Flarakos J, Du Y, Gu H, Wang L, Einolf HJ, Chun DY, Zhu B, Alexander N, Natrillo A, Hanna I, Ting L, Zhou W, Dole K, Sun H, Kovacs SJ, Stein DS, Tanaka SK, Villano S, Mangold JB. August 2016. Disposition, metabolism, and drug-drug interaction properties of omadacycline. Xenobiotica doi:10.1080/00498254.2016.1213465. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

