Sustained Nitric Oxide-Releasing Nanoparticles Interfere with Methicillin-Resistant Staphylococcus aureus Adhesion and Biofilm Formation in a Rat Central Venous Catheter Model
- PMID: 27821454
- PMCID: PMC5192117
- DOI: 10.1128/AAC.02020-16
Sustained Nitric Oxide-Releasing Nanoparticles Interfere with Methicillin-Resistant Staphylococcus aureus Adhesion and Biofilm Formation in a Rat Central Venous Catheter Model
Abstract
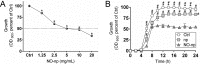
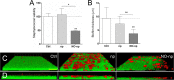
Staphylococcus aureus is frequently isolated in the setting of infections of indwelling medical devices, which are mediated by the microbe's ability to form biofilms on a variety of surfaces. Biofilm-embedded bacteria are more resistant to antimicrobial agents than their planktonic counterparts and often cause chronic infections and sepsis, particularly in patients with prolonged hospitalizations. In this study, we demonstrate that sustained nitric oxide-releasing nanoparticles (NO-np) interfere with S. aureus adhesion and prevent biofilm formation on a rat central venous catheter (CVC) model of infection. Confocal and scanning electron microscopy showed that NO-np-treated staphylococcal biofilms displayed considerably reduced thicknesses and bacterial numbers compared to those of control biofilms in vitro and in vivo, respectively. Although both phenotypes, planktonic and biofilm-associated staphylococci, of multiple clinical strains were susceptible to NO-np, bacteria within biofilms were more resistant to killing than their planktonic counterparts. Furthermore, chitosan, a biopolymer found in the exoskeleton of crustaceans and structurally integrated into the nanoparticles, seems to add considerable antimicrobial activity to the technology. Our findings suggest promising development and translational potential of NO-np for use as a prophylactic or therapeutic against bacterial biofilms on CVCs and other medical devices.
Keywords: Staphylococcus aureus; antimicrobials; biofilms; nanoparticles; nitric oxide.
Copyright © 2016 American Society for Microbiology.
Figures



Similar articles
-
Sustained Nitric Oxide-Releasing Nanoparticles Induce Cell Death in Candida albicans Yeast and Hyphal Cells, Preventing Biofilm Formation In Vitro and in a Rodent Central Venous Catheter Model.Antimicrob Agents Chemother. 2016 Mar 25;60(4):2185-94. doi: 10.1128/AAC.02659-15. Print 2016 Apr. Antimicrob Agents Chemother. 2016. PMID: 26810653 Free PMC article.
-
Molecular analysis, biofilm formation, and susceptibility of methicillin-resistant Staphylococcus aureus strains causing community- and health care-associated infections in central venous catheters.Rev Soc Bras Med Trop. 2018 Sep-Oct;51(5):603-609. doi: 10.1590/0037-8682-0373-2017. Rev Soc Bras Med Trop. 2018. PMID: 30304265
-
[Investigation of biofilm-associated antibiotic susceptibilities of methicillin-resistant staphylococci isolated from catheter-related nosocomial infections].Mikrobiyol Bul. 2013 Jul;47(3):401-16. doi: 10.5578/mb.5637. Mikrobiyol Bul. 2013. PMID: 23971919 Turkish.
-
Effect of vancomycin-coated tympanostomy tubes on methicillin-resistant Staphylococcus aureus biofilm formation: in vitro study.J Laryngol Otol. 2010 Jun;124(6):594-8. doi: 10.1017/S0022215109992672. Epub 2010 Jan 8. J Laryngol Otol. 2010. PMID: 20056010 Review.
-
Suppression of biofilm related, device-associated infections by staphylococcal quorum sensing inhibitors.Int J Artif Organs. 2008 Sep;31(9):761-70. doi: 10.1177/039139880803100903. Int J Artif Organs. 2008. PMID: 18924087 Review.
Cited by
-
Metal nanoparticles as inhibitors of enzymes and toxins of multidrug-resistant Staphylococcus aureus.Infect Med (Beijing). 2023 Nov 21;2(4):294-307. doi: 10.1016/j.imj.2023.11.006. eCollection 2023 Dec. Infect Med (Beijing). 2023. PMID: 38205183 Free PMC article. Review.
-
Assessing and improving the biocompatibility of microfluidic artificial lungs.Acta Biomater. 2020 Aug;112:190-201. doi: 10.1016/j.actbio.2020.05.008. Epub 2020 May 17. Acta Biomater. 2020. PMID: 32434076 Free PMC article.
-
Biofilm dispersion.Nat Rev Microbiol. 2020 Oct;18(10):571-586. doi: 10.1038/s41579-020-0385-0. Epub 2020 Jun 12. Nat Rev Microbiol. 2020. PMID: 32533131 Free PMC article. Review.
-
Antibacterial gas therapy: Strategies, advances, and prospects.Bioact Mater. 2022 Nov 11;23:129-155. doi: 10.1016/j.bioactmat.2022.10.008. eCollection 2023 May. Bioact Mater. 2022. PMID: 36406249 Free PMC article. Review.
-
Nanomaterials for Treating Bacterial Biofilms on Implantable Medical Devices.Nanomaterials (Basel). 2020 Nov 13;10(11):2253. doi: 10.3390/nano10112253. Nanomaterials (Basel). 2020. PMID: 33203046 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

