Heterogeneous Stromal Signaling within the Tumor Microenvironment Controls the Metastasis of Pancreatic Cancer
- PMID: 27821486
- PMCID: PMC5245778
- DOI: 10.1158/0008-5472.CAN-16-1383
Heterogeneous Stromal Signaling within the Tumor Microenvironment Controls the Metastasis of Pancreatic Cancer
Abstract
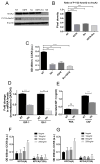
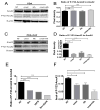
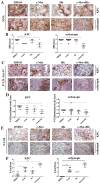
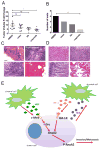
Understanding how stromal signals regulate the development of pancreatic ductal adenocarcinoma (PDAC) may suggest novel therapeutic interventions in this disease. In this study, we assessed the metastatic role of stromal signals suggested to be important in the PDAC microenvironment. Src and IGF-1R phosphorylated the prometastatic molecule Annexin A2 (AnxA2) at Y23 and Y333 in response to stromal signals HGF and IGF-1, respectively, and IGF-1 expression was regulated by the Sonic Hedgehog (Shh) pathway. Both Shh and HGF were heterogeneously expressed in PDAC stroma, and only dual inhibition of these pathways could significantly suppress AnxA2 phosphorylation, PDAC growth, and metastasis. Taken together, our results illuminate tumor-stromal interactions, which drive metastasis, and provide a mechanism-based rationale for a stroma-directed therapy for PDAC. Cancer Res; 77(1); 41-52. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
of Potential Conflict of Interests: No relevant conflicts of interest to disclose.
Figures


References
-
- Chu GC, Kimmelman AC, Hezel AF, DePinho RA. Stromal biology of pancreatic cancer. Journal of cellular biochemistry. 2007;101(4):887–907. - PubMed
-
- Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425(6960):846–51. - PubMed
-
- Shinozaki S, Ohnishi H, Hama K, Kita H, Yamamoto H, Osawa H, et al. Indian hedgehog promotes the migration of rat activated pancreatic stellate cells by increasing membrane type-1 matrix metalloproteinase on the plasma membrane. Journal of cellular physiology. 2008;216(1):38–46. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

